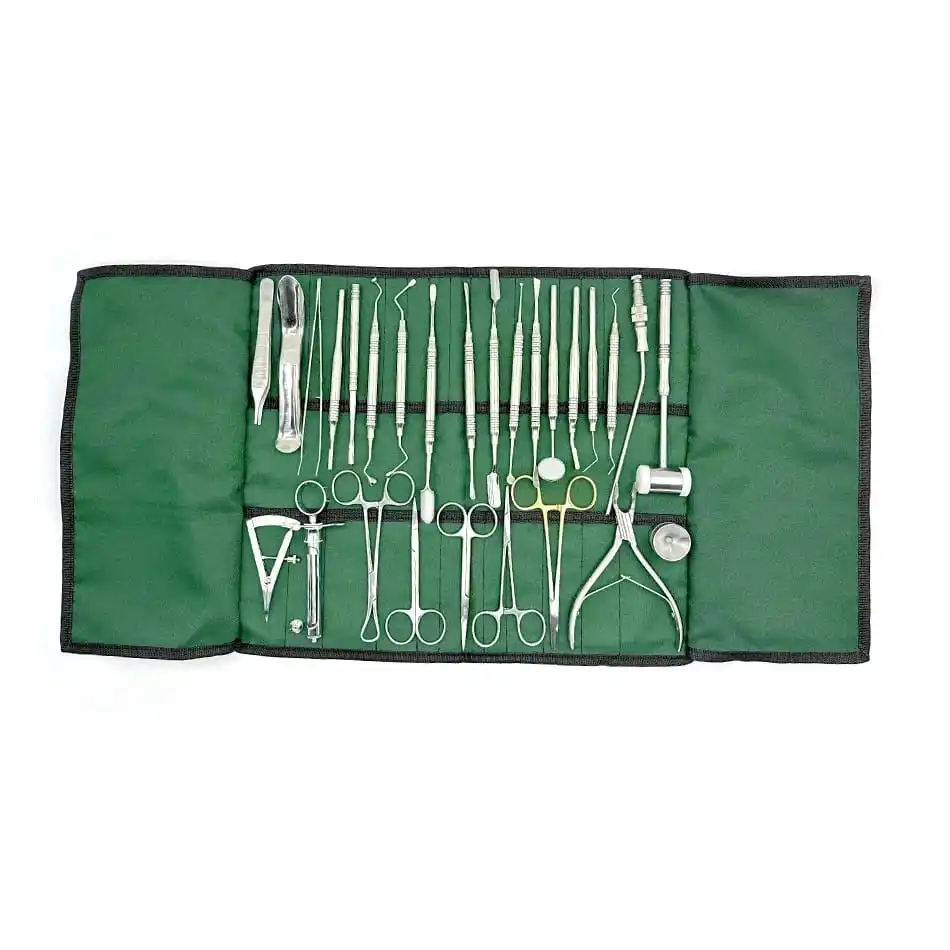
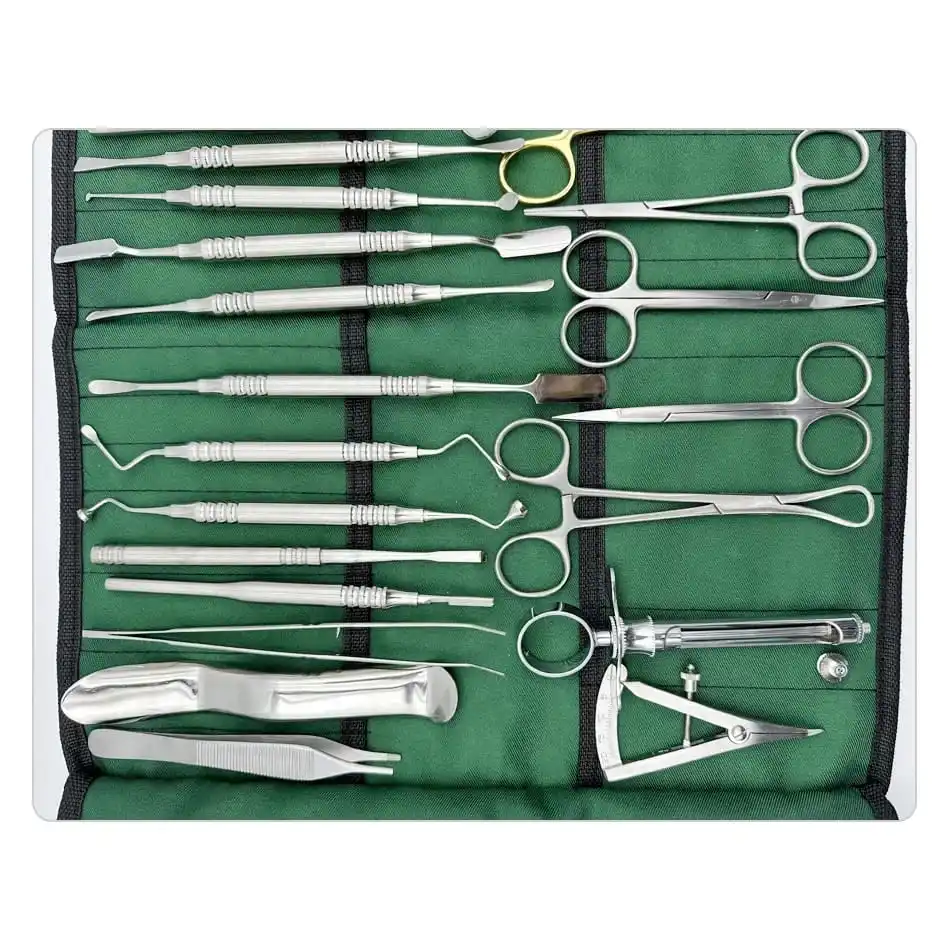
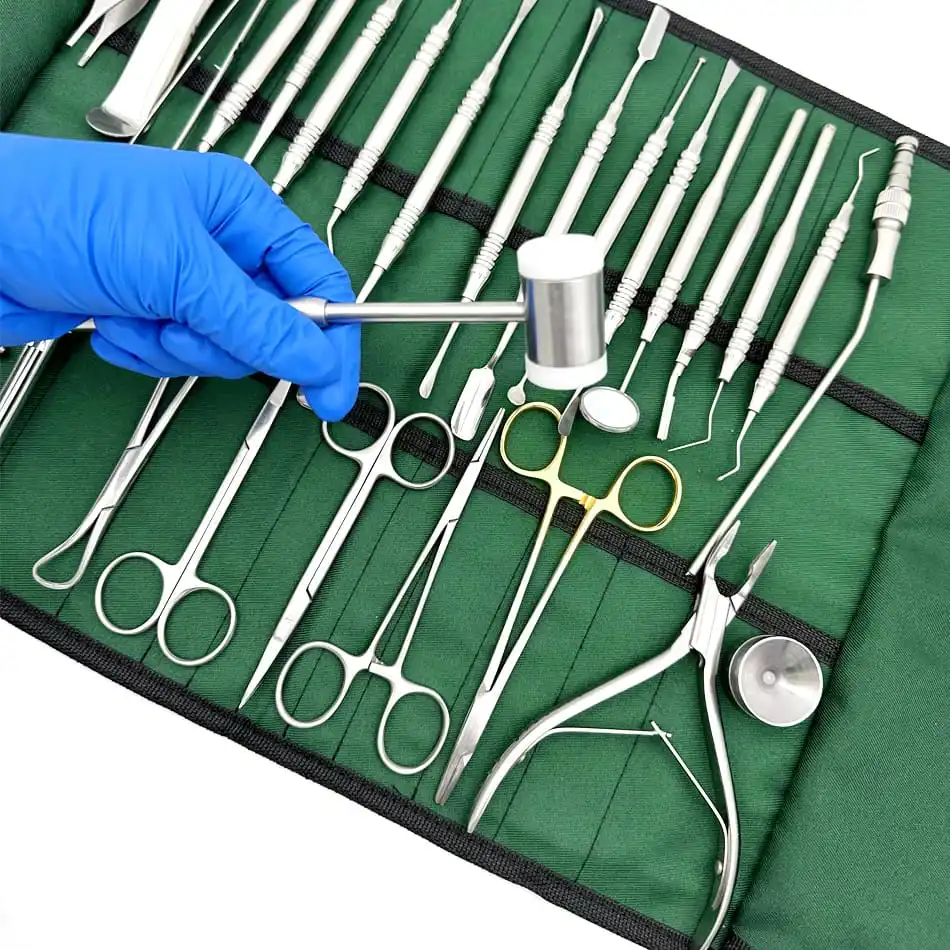
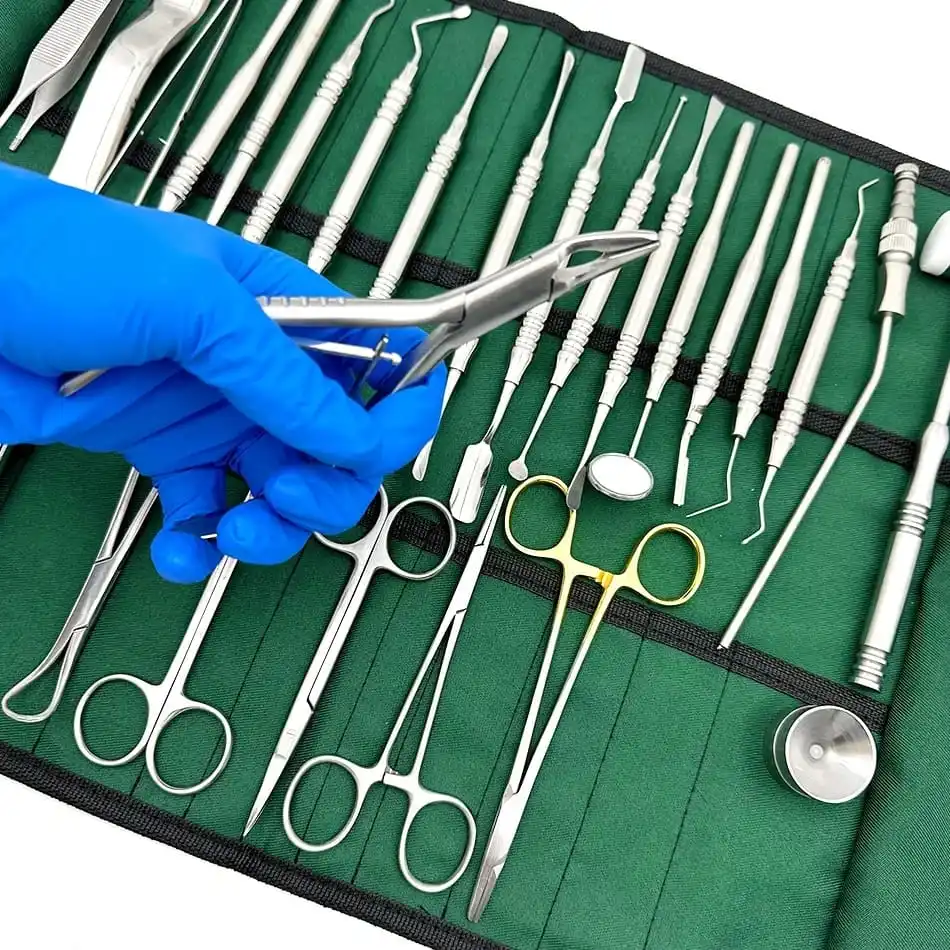
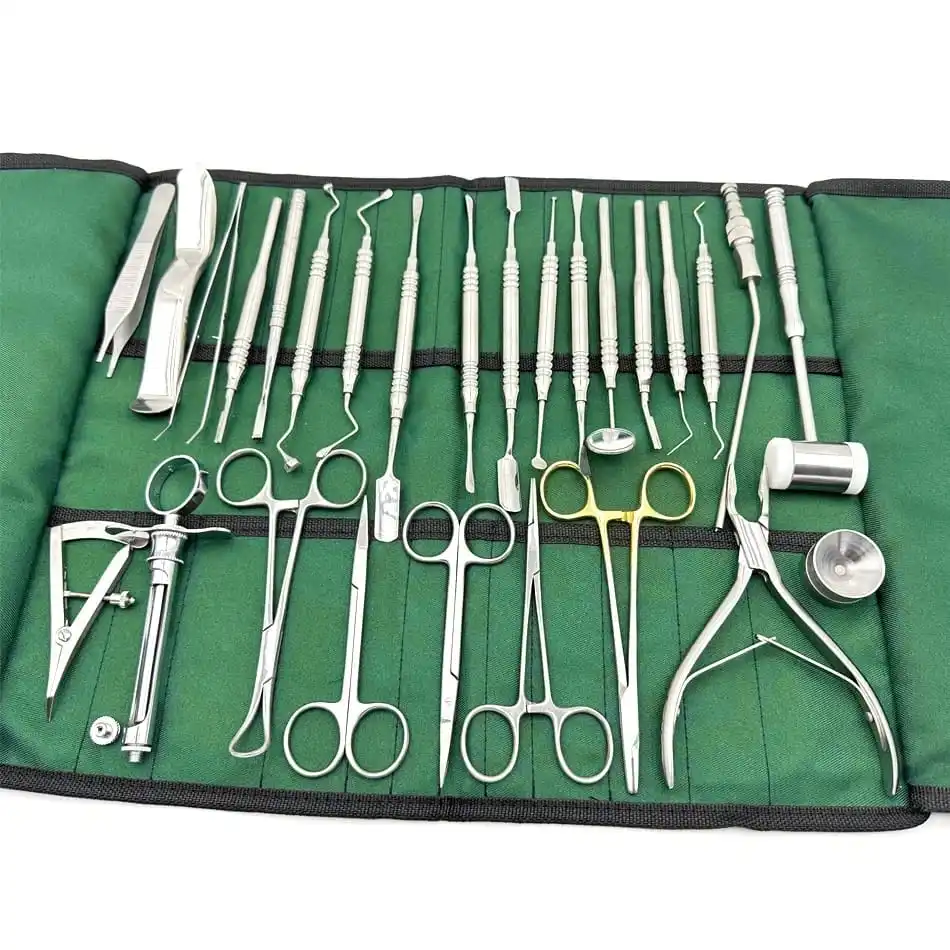
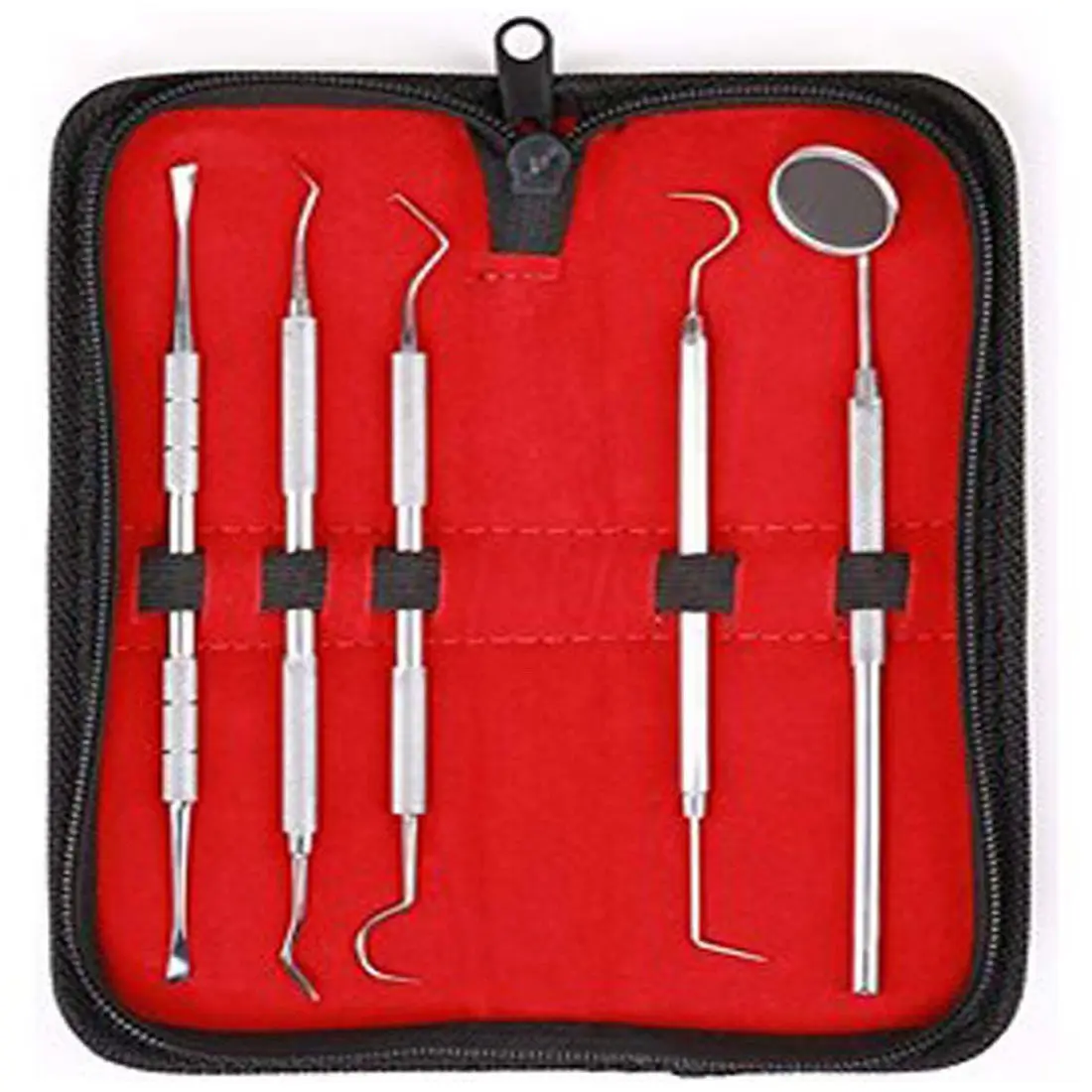
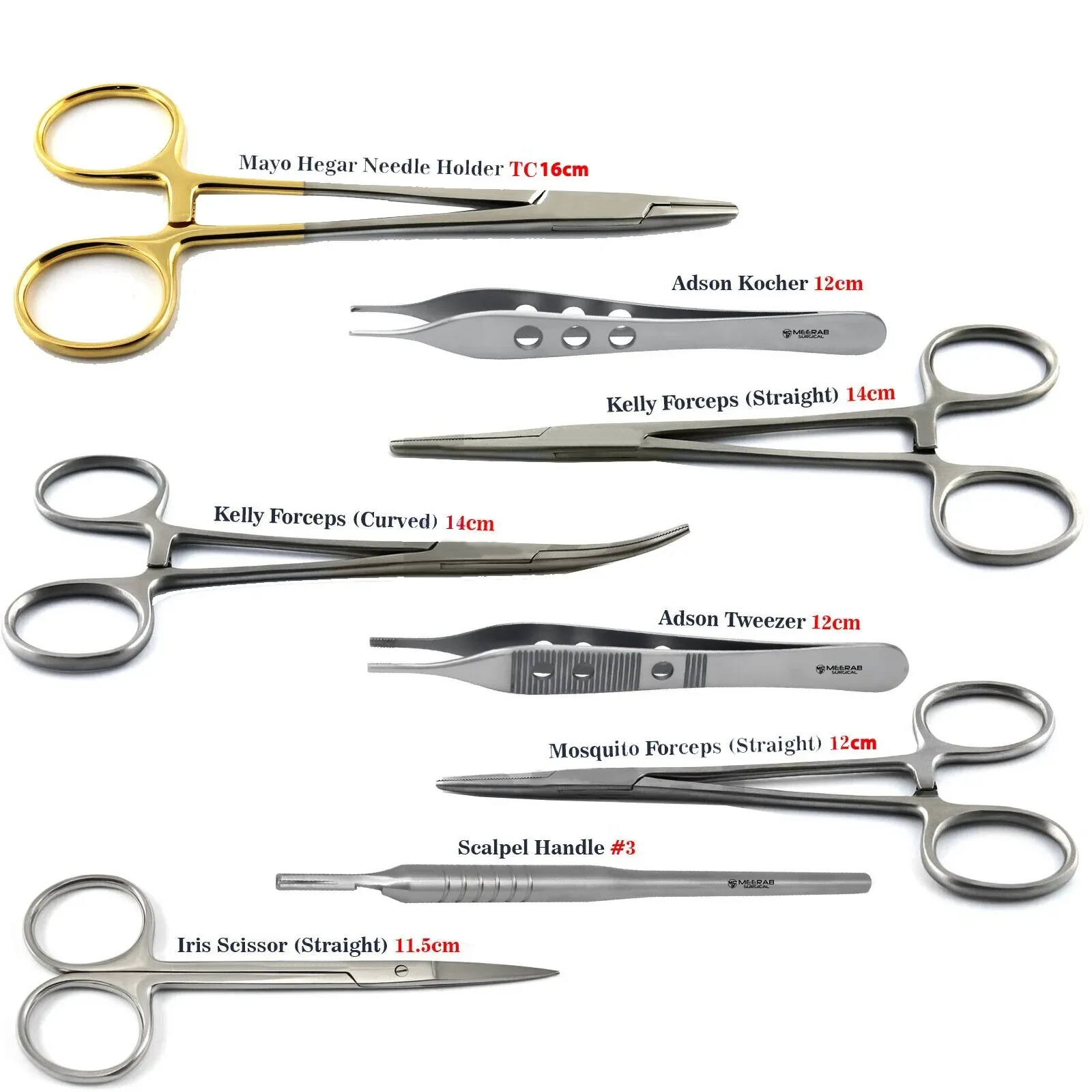
CE Certified 26 Pcs Orthodontic Dental Surgical Instruments Kit German Stainless Steel Manual Implant Surgery Tools Sambrialmed
- Category: >>>
- Supplier: SAMBRIALMED INTERNATIONAL
Share on (11000016002646):
Product Overview
Description
Product Description
26 Pcs / Set Dental Tool Kit Implant Surgery Instruments Set
TECHNICAL SPECIFICATIONS:
* Material: German Stainless steel
* Rusting Prevention Procedure: Passivated
* Ultrasonic Cleaned: Yes
* Dull-Polished: Yes
* Tests Performed: Boil Test, Performance Test, Shape Test
* Packing: Individually Packed
* Grade: High
* Sterility: Non-Sterile
* QC Passed: Yes
SALIENT FEATURES:
* Surgical grade German Stainless Steel.
* Made with superior quality clinical grade and autoclave able Material.
* Hand Matt Polished to avoid reflections and durability.
* Completely conformed to CE and ISO standards.
* Corrosion resistance, no chrome plating no risk of plating peeling off.
* Easy instruments care, all standard sterilization procedure applicable.
* 100% satisfaction or your Money Back.
* Material: German Stainless steel
* Rusting Prevention Procedure: Passivated
* Ultrasonic Cleaned: Yes
* Dull-Polished: Yes
* Tests Performed: Boil Test, Performance Test, Shape Test
* Packing: Individually Packed
* Grade: High
* Sterility: Non-Sterile
* QC Passed: Yes
SALIENT FEATURES:
* Surgical grade German Stainless Steel.
* Made with superior quality clinical grade and autoclave able Material.
* Hand Matt Polished to avoid reflections and durability.
* Completely conformed to CE and ISO standards.
* Corrosion resistance, no chrome plating no risk of plating peeling off.
* Easy instruments care, all standard sterilization procedure applicable.
* 100% satisfaction or your Money Back.
RELATED IMAGES
Specifications
Place of Origin | Pakistan |
Brand Name | Sambrialmed International |
Model Number | SI-DT-02 |
Power Source | Manual |
Warranty | 1 Year |
After-sale Service | Return and Replacement |
Material | German Stainless Steel |
Shelf Life | 1 years |
Quality Certification | CE |
Instrument classification | Class I |
Safety standard | None |
Product name | Edentulous Perforated |
MOQ | 10 Piece |
Usage | Dental Implant Surgery |
Keywords | Stainless Steel Operating Surgical Instruments |
Feature | Reusable Surgery Instruments |
Color | Customized Colour |
Packing & Delivery



To better ensure the safety of your goods, professional, environmentally friendly, convenient and efficient packaging services will be provided.
Company Profile

(ISO 13485:2016)
Manufacturer of Non-Active Surgical, Dental Instruments and hollowware
(ISO 9001:2015)
Manufacturer of Surgical, Dental, Veterinary Instruments and hollowware
(cGMP 21 CFR Part 820)
Manufacturer of Non-Active Surgical, Dental Instruments and hollowware
Sambrialmed International, abide by all the applicable regulatory national & international laws related to the
welfare of employees as well as occupational health and safety management system.
OUR GOALS
HIGH QUALITY INSTRUMENTS COMPETITIVE PRICES
TIMELY DELIVERY
CONSISTENCY OF PRODUCTS
Sambrialmed International® has made the “Strategic Business Decision” to develop and implement an effective Quality
Management Systems (QMS) for Medical Devices across all functions of the Company. Organization involved in one or more stages of the life cycle of a medical device i.e. production, storage and distribution. Suppliers or other external parties providing product (e.g. raw materials, components, subassemblies, semi-finished medical devices and process services, calibration services, distribution services, maintenance services) to organizations can also use the guidelines and principles in this QMS Manual.
FAQ

1. who are we?
We are based in Florida, United States, start from 2010,sell to South America(30.00%),Central America(20.00%),North
America(20.00%),Eastern Europe(10.00%),Northern Europe(10.00%),South Asia(5.00%),Mid East(5.00%). There are total about 51-100 people in our office.
2. how can we guarantee quality?
Always a pre-production sample before mass production; Always final Inspection before shipment;
3.what can you buy from us?
Dental Instruments,Orthopaedic Instruments, General Instruments, Plastic Surgery Instruments , Ent Instruments, VATs Instruments
4. why should you buy from us not from other suppliers?
Every Sambrialmed international instrument is produced to our rigid quality control standards and is unconditionally guaranteed against possible defects in material or workmanship. Any instrument proven defective in workmanship ..
5. what services can we provide?
Accepted Delivery Terms: FOB,CFR,CIF,EXW,CIP,CPT,DDP,DDU;
Accepted Payment Currency:USD,EUR,GBP;
Accepted Payment Type: T/T,L/C,MoneyGram,Credit Card,PayPal,Western Union,Cash;
Language Spoken:English,Spanish,German,Hindi
We are based in Florida, United States, start from 2010,sell to South America(30.00%),Central America(20.00%),North
America(20.00%),Eastern Europe(10.00%),Northern Europe(10.00%),South Asia(5.00%),Mid East(5.00%). There are total about 51-100 people in our office.
2. how can we guarantee quality?
Always a pre-production sample before mass production; Always final Inspection before shipment;
3.what can you buy from us?
Dental Instruments,Orthopaedic Instruments, General Instruments, Plastic Surgery Instruments , Ent Instruments, VATs Instruments
4. why should you buy from us not from other suppliers?
Every Sambrialmed international instrument is produced to our rigid quality control standards and is unconditionally guaranteed against possible defects in material or workmanship. Any instrument proven defective in workmanship ..
5. what services can we provide?
Accepted Delivery Terms: FOB,CFR,CIF,EXW,CIP,CPT,DDP,DDU;
Accepted Payment Currency:USD,EUR,GBP;
Accepted Payment Type: T/T,L/C,MoneyGram,Credit Card,PayPal,Western Union,Cash;
Language Spoken:English,Spanish,German,Hindi
We Recommend
New Arrivals
New products from manufacturers at wholesale prices