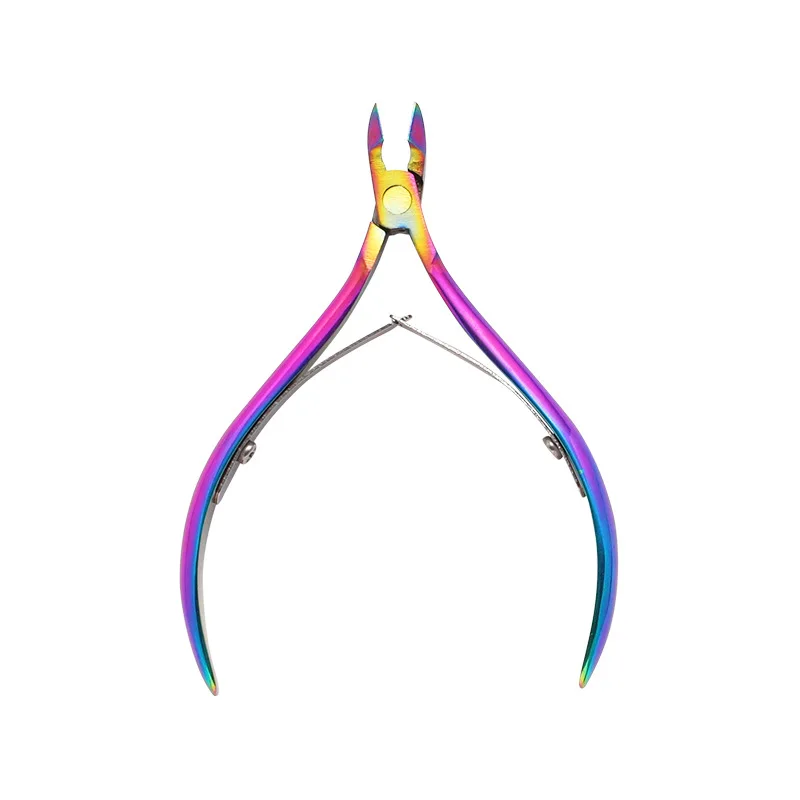
Nail Cuticle Nipper Scissors Stainless Steel Manicure Colorful Clipper Dead Skin Remover Pedicure Eagle Beak Pliers
- Category: >>>
- Supplier: SAMBRIALMED INTERNATIONAL
Share on (11000019055530):
Product Overview
Description
Product Description
Specifications
Brand Name | Sambrialmed International |
Blade Material | Stainless Steel |
Product name | Nail Clipper |
MOQ | 10 Pieces |
Type | Nail Care Tool |
Size | 6.5"/9"/9.5" |
Color | Customized Colour |
Packing & Delivery



To better ensure the safety of your goods, professional, environmentally friendly, convenient and efficient packaging services will be provided.
Company Profile

(ISO 13485:2016)
Manufacturer of Non-Active Surgical, Dental Instruments and hollowware
(ISO 9001:2015)
Manufacturer of Surgical, Dental, Veterinary Instruments and hollowware
(cGMP 21 CFR Part 820)
Manufacturer of Non-Active Surgical, Dental Instruments and hollowware
Sambrialmed International, abide by all the applicable regulatory national & international laws related to the
welfare of employees as well as occupational health and safety management system.
OUR GOALS
HIGH QUALITY INSTRUMENTS COMPETITIVE PRICES
TIMELY DELIVERY
CONSISTENCY OF PRODUCTS
Sambrialmed International® has made the “Strategic Business Decision” to develop and implement an effective Quality
Management Systems (QMS) for Medical Devices across all functions of the Company. Organization involved in one or more stages of the life cycle of a medical device i.e. production, storage and distribution. Suppliers or other external parties providing product (e.g. raw materials, components, subassemblies, semi-finished medical devices and process services, calibration services, distribution services, maintenance services) to organizations can also use the guidelines and principles in this QMS Manual.
FAQ

Q:What is quality warranty ?
Ans.We produce German grade quality ,high quality stainless steel made, we offer free replacement warranty in case of any qualitycomplain, ISO CE certificates are available for more satisfaction.
Q:How i can proceed my order ?
Ans:Just tell us how many pcs do you need and where to ship? we will give you price and after price confirmation we will send you an offical invoice for payment.
Q:Can i put my logo or name ?
Ans:Yes we offer this service free of cost by laser machine, free your loog or name stamp?
Q:What are payment terms?
Ans:For small order value like 2000 US$ or less we accept 100% advance, for big orders value we can negotiate the payment terms.
Q:What are payment methods?
Ans:We accept TT Bank,Pay Pal,Credit Card,Western Union ,Money Gram & Cash.
Q:What is delivery time?
Ans:After order and payment, we take 10 days minimum for a small order and for the large orders minimum we takes 4 weeks.
Q:What is shipping method?
Ans:We use DHL, FEDEX, UPS,TNT, EMS, Air Cargo and Sea Cargo
Q:How we can trust on this company?
Ans:We are professional registered manufacturing and export company, alibaba gold supplier, all legal documents are available for more satisfaction.
Note:The listed price is not the final price, the final price will depend on quantity.
Ans.We produce German grade quality ,high quality stainless steel made, we offer free replacement warranty in case of any qualitycomplain, ISO CE certificates are available for more satisfaction.
Q:How i can proceed my order ?
Ans:Just tell us how many pcs do you need and where to ship? we will give you price and after price confirmation we will send you an offical invoice for payment.
Q:Can i put my logo or name ?
Ans:Yes we offer this service free of cost by laser machine, free your loog or name stamp?
Q:What are payment terms?
Ans:For small order value like 2000 US$ or less we accept 100% advance, for big orders value we can negotiate the payment terms.
Q:What are payment methods?
Ans:We accept TT Bank,Pay Pal,Credit Card,Western Union ,Money Gram & Cash.
Q:What is delivery time?
Ans:After order and payment, we take 10 days minimum for a small order and for the large orders minimum we takes 4 weeks.
Q:What is shipping method?
Ans:We use DHL, FEDEX, UPS,TNT, EMS, Air Cargo and Sea Cargo
Q:How we can trust on this company?
Ans:We are professional registered manufacturing and export company, alibaba gold supplier, all legal documents are available for more satisfaction.
Note:The listed price is not the final price, the final price will depend on quantity.
We Recommend
New Arrivals
New products from manufacturers at wholesale prices