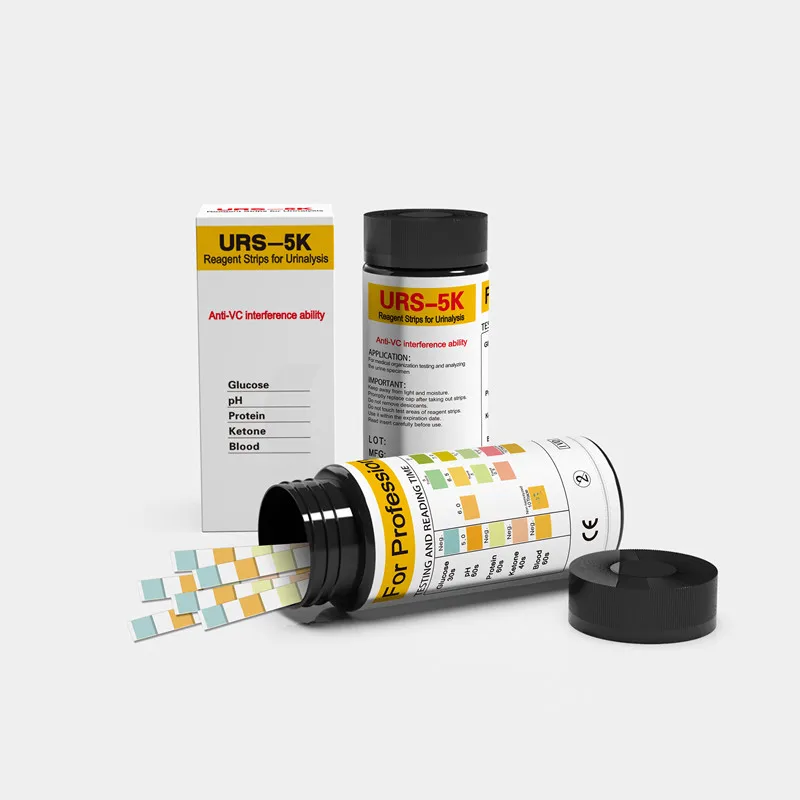
URS 5K мочевые тест полоски экспресс тест набор для домашнего использования клиники и больницы
- Категория: >>>
- Поставщик: Changchun Merydi Bio-Tech Co. Ltd.
Сохранить в закладки 1600322319070:
Описание и отзывы
Характеристики
Product Description
This test is slightly more sensitive to free hemoglobin and myoglobin than to intact erythrocytes. The test is generally capable of detecting 0.015-mg/dl free hemoglobin or 5 to 10 intact red blood cells per ml of urine. The sensitivity may he reduced in urine with high specific gravity and ascorbic acid content. The appearance of green spots on the reagent test area indicates the presence of intact erythrocytes in the urine.





Glucose
This test is based on a sequential enzyme reaction. First, glucose oxidase catalyzes the formation of gluconic acid and hydrogen peroxide from the oxidation of glucose. A second enzyme, peroxidase, catalyzes the reaction of hydrogen peroxide with potassium iodide chromogen to oxidize the chromogen to colors ranging from blue through greenish-brown, and brown to dark-brown.
Reactivity of the test decreases as the specific gravity and/or pH of urine increases, and may also vary with temperature.
Ascorbic acid (more than 50 mg/dl) and ketone bodies (more than 40 mg/dl) may cause a false negative result for a specimen containing a small amount of glucose (100 mg/dl). However, the combinations of such ketone levels and low glucose levels are metabolically improbable.
Reactivity of the test decreases as the specific gravity and/or pH of urine increases, and may also vary with temperature.
Ascorbic acid (more than 50 mg/dl) and ketone bodies (more than 40 mg/dl) may cause a false negative result for a specimen containing a small amount of glucose (100 mg/dl). However, the combinations of such ketone levels and low glucose levels are metabolically improbable.
pH
This test is based on double indicators (methyl red and bromothymol blue), which give a broad range of colors covering the entire urinary pH range. Colors range from orange through greenish-yellow and green to blue.
This test indicates the pH values within the range of 5 to 9.
Certain drugs, such as those used for hypertension and heart diseases (acetazolamides) may cause an alkaline urine. Excessive urine on the test strip may wash the acid buffer from the neighboring protein reagent onto the pH area and change the pH reading to an acid pH although the urine being tested may originally have been neutral or alkaline. An accurate reading may be influenced by slight variations of pigments in the urine.
This test is based on double indicators (methyl red and bromothymol blue), which give a broad range of colors covering the entire urinary pH range. Colors range from orange through greenish-yellow and green to blue.
This test indicates the pH values within the range of 5 to 9.
Certain drugs, such as those used for hypertension and heart diseases (acetazolamides) may cause an alkaline urine. Excessive urine on the test strip may wash the acid buffer from the neighboring protein reagent onto the pH area and change the pH reading to an acid pH although the urine being tested may originally have been neutral or alkaline. An accurate reading may be influenced by slight variations of pigments in the urine.
Protein
This test is based on the color change of the indicator tetrabromophenol blue. A positive reaction is indicated by a color change from yellow through green and then to greenish-blue.
The minimum sensitivity of this test is 10 mg/dl of protein in urine. Highly buffered alkaline urines (pH 8.5) may give false
negative results. The interpretation of results is also difficult in turbid urine specimens.
This test is based on the color change of the indicator tetrabromophenol blue. A positive reaction is indicated by a color change from yellow through green and then to greenish-blue.
The minimum sensitivity of this test is 10 mg/dl of protein in urine. Highly buffered alkaline urines (pH 8.5) may give false
negative results. The interpretation of results is also difficult in turbid urine specimens.
Ketone
This test is based on the reaction of acetoacetic acid in the urine with nitroprusside. The resulting color ranges from tan when no reaction takes place, to purple for a positive reaction. Normal urine specimens ordinarily yield negative results with this reagent.
False positive results may occur with highly pigmented urine specimens or those containing large amounts of levodopa metabolites.
This test is based on the reaction of acetoacetic acid in the urine with nitroprusside. The resulting color ranges from tan when no reaction takes place, to purple for a positive reaction. Normal urine specimens ordinarily yield negative results with this reagent.
False positive results may occur with highly pigmented urine specimens or those containing large amounts of levodopa metabolites.
Blood
This test is based on the pseudoperoxidase activity of hemoglobin which catalyzes the reaction of 3,3'5,5'-tetramethylbenzidine and buffered organic peroxide, 2,5-dimethylhexane-2,5-dihydroperoxide. The resulting color ranges from, greenish-yellow through bluish-green to dark blue.
A false positive test result can sometimes occur when bacteria are present in the urine. Ascorbic acid or protein may reduce the reactivity of the blood test. Strong oxidizing substances such as hypochlorites may produce a false positive result. Urine from menstruating females often, but not always, yield positive results.
This test is based on the pseudoperoxidase activity of hemoglobin which catalyzes the reaction of 3,3'5,5'-tetramethylbenzidine and buffered organic peroxide, 2,5-dimethylhexane-2,5-dihydroperoxide. The resulting color ranges from, greenish-yellow through bluish-green to dark blue.
A false positive test result can sometimes occur when bacteria are present in the urine. Ascorbic acid or protein may reduce the reactivity of the blood test. Strong oxidizing substances such as hypochlorites may produce a false positive result. Urine from menstruating females often, but not always, yield positive results.
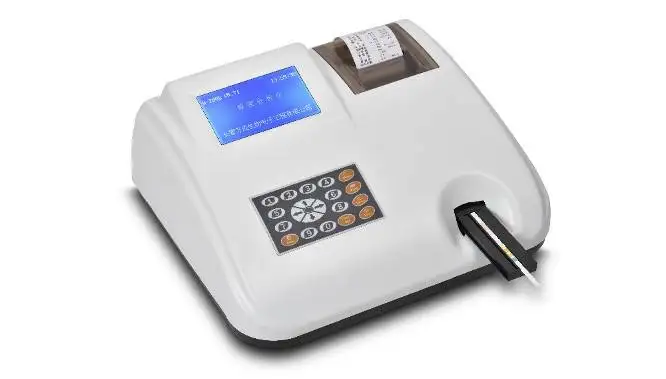
Specification
item | value |
Type | Urine Analysis System |
Brand Name | Merydi |
Model Number | URS-5K |
Place of Origin | China |
Province | Jilin |
Instrument classification | Class II |
Warranty | 2 years |
After-sale Service | Online technical support |
Product name | Urine Test Strips |
Function | Detecting Body Health |
Certificate | CE ISO |
MOQ | 10 barrels |
Power Supply | Manual |
Sample | Avaliable |
Accuracy | 99.96% |
Test Time | 30~60s |
Package | OEM/Neutral/Brand |
Storage | Dry and Sealed |
Packing & Delivery


25/50/100/150/200/250 strips per barrel
100/200 barrels per carton
7-18 Kg per carton
100/200 barrels per carton
7-18 Kg per carton
Company Profile




CE Certificate
ISO13485
Changchun Merydi Bio-tech Co., Ltd was set up in 2008 and specializes in the research and development, production and sales of Medical Appliances and Reagents.
We are a professional manufacturer of Medical Urine Test Strips, Urine analyzer, Hematology analyzer and hematology reagent. As for the Medical Urine Analyzers, we provide Semi-automatic urine analyzer. As for the hematology analyzer, we provide 3-part and 5 part diff hematology analyzer CBC-6000.Our core products Urine Test Strips include Urine Test Strips - 1, 2, 3, 4, 5, 10, 11 and 12 parameters, drug test strip, HIV test strip, C reaction diagnostic test strip etc. All the products we provide come with good quality and competitive price.
We are a professional manufacturer of Medical Urine Test Strips, Urine analyzer, Hematology analyzer and hematology reagent. As for the Medical Urine Analyzers, we provide Semi-automatic urine analyzer. As for the hematology analyzer, we provide 3-part and 5 part diff hematology analyzer CBC-6000.Our core products Urine Test Strips include Urine Test Strips - 1, 2, 3, 4, 5, 10, 11 and 12 parameters, drug test strip, HIV test strip, C reaction diagnostic test strip etc. All the products we provide come with good quality and competitive price.
FAQ
1. who are we?
We are based in Jilin, China, start from 2008,sell to North America(51.00%),Domestic Market(24.00%),Western Europe(9.00%),Northern Europe(5.00%),South America(2.00%),Eastern Europe(00.00%),Southeast Asia(00.00%),Africa(00.00%),Oceania(00.00%),Mid East(00.00%),Eastern Asia(00.00%),Central America(00.00%),Southern Europe(00.00%),South Asia(00.00%). There are total about 11-50 people in our office.
2. how can we guarantee quality?
Always a pre-production sample before mass production;
Always final Inspection before shipment;
3.what can you buy from us?
Urine (Saliva) Test Strip,Rapid test Strip,Water Test Strip,Urine Analyzer,Hematology Analyzer
4. why should you buy from us not from other suppliers?
We are a professional manufacturer of Medical Urine Test Strips , Urine analyzer, Hematology analyzer and hematology reagent etc. We have more than 15 R&D staff.
5. what services can we provide?
Accepted Delivery Terms: CFR,CIF;
Accepted Payment Currency:USD;
Accepted Payment Type: T/T,MoneyGram,PayPal,Western Union;
Language Spoken:English,Chinese,Russian
We are based in Jilin, China, start from 2008,sell to North America(51.00%),Domestic Market(24.00%),Western Europe(9.00%),Northern Europe(5.00%),South America(2.00%),Eastern Europe(00.00%),Southeast Asia(00.00%),Africa(00.00%),Oceania(00.00%),Mid East(00.00%),Eastern Asia(00.00%),Central America(00.00%),Southern Europe(00.00%),South Asia(00.00%). There are total about 11-50 people in our office.
2. how can we guarantee quality?
Always a pre-production sample before mass production;
Always final Inspection before shipment;
3.what can you buy from us?
Urine (Saliva) Test Strip,Rapid test Strip,Water Test Strip,Urine Analyzer,Hematology Analyzer
4. why should you buy from us not from other suppliers?
We are a professional manufacturer of Medical Urine Test Strips , Urine analyzer, Hematology analyzer and hematology reagent etc. We have more than 15 R&D staff.
5. what services can we provide?
Accepted Delivery Terms: CFR,CIF;
Accepted Payment Currency:USD;
Accepted Payment Type: T/T,MoneyGram,PayPal,Western Union;
Language Spoken:English,Chinese,Russian

Похожие товары
Высококачественный Взрывозащищенный соединитель Соединительный фитинг для труб из углеродистой стали Weldolet
Высококачественный Взрывозащищенный соединитель Соединительный фитинг для труб из углеродистой стали Weldolet
1,80 $ - 2,00 $
SW-7000 использование брошюра для промышленного регулятора регулятор температуры
Бесплатная доставка в США, гладкие шелковые трусики-стринги, женские атласные стринги
0,79 $ - 0,89 $
Высококачественный волоконно-армированный Стекловолоконный прочный элемент для волоконно-оптических кабелей, производственный стержень
2,50 $ - 5,00 $
Высококачественная связка проводов, новые инструменты, строительный инструмент, портативная автоматическая машина для подвязки арматуры
Компоненты Стартера Якорь A-BPS31015 ВОЛГА ГАЗ ЛАДА ВАЗ КЗАТЭ КАТЭК 57 Коригинальный
Новые поступления
Новинки товаров от производителей по оптовым ценам
Hantek щупы осциллографа автоматический Тестовый Кабель Ht30a Bnc к банану двойная Банановая головка многоцелевое автомобильное
3,10-3,44 $
Горячая распродажа ручная работа традиционная деревянная урна для домашних животных квадратная коробка золы собак и кошек персонализированная Лучшая цена
Промышленная серия CL Бесшумная цепь стальная зубчатая Фланцевая контактная звездочка соединяющая бесшумные цепи
1-1,50 $
Irdmco экологически чистый минималистичный прямоугольный деревянный ящик для хранения стеллаж поделок и детских игрушек Домашняя мебель гостиной
Технология сенсорных перчаток и аксессуары для детей от дождя холода M725132-334
1,07-1,40 $
GY6 6 Pin AC гоночный CDI для CG125 CG250 двигателя Dirt Pit Bike ATV Quad
Парик из синтетического волокна для косплея
9,85-12,66 $
Прямая Продажа с фабрики 65 см Модернизированный подвесный стерео голографический проектор светодиодный вентилятор высокой четкости экран 3D дисплей
155-185 $