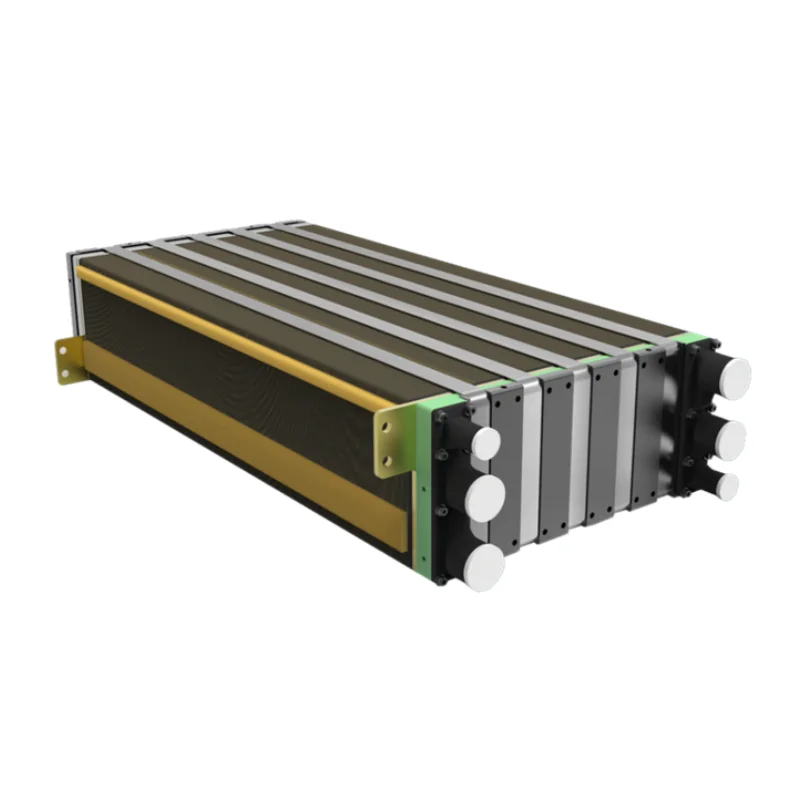
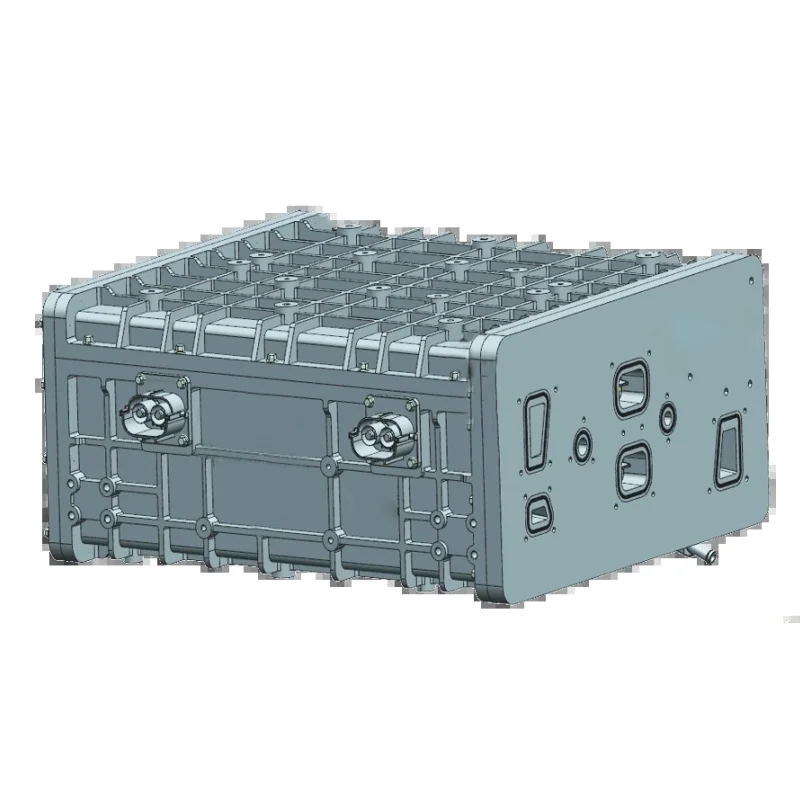
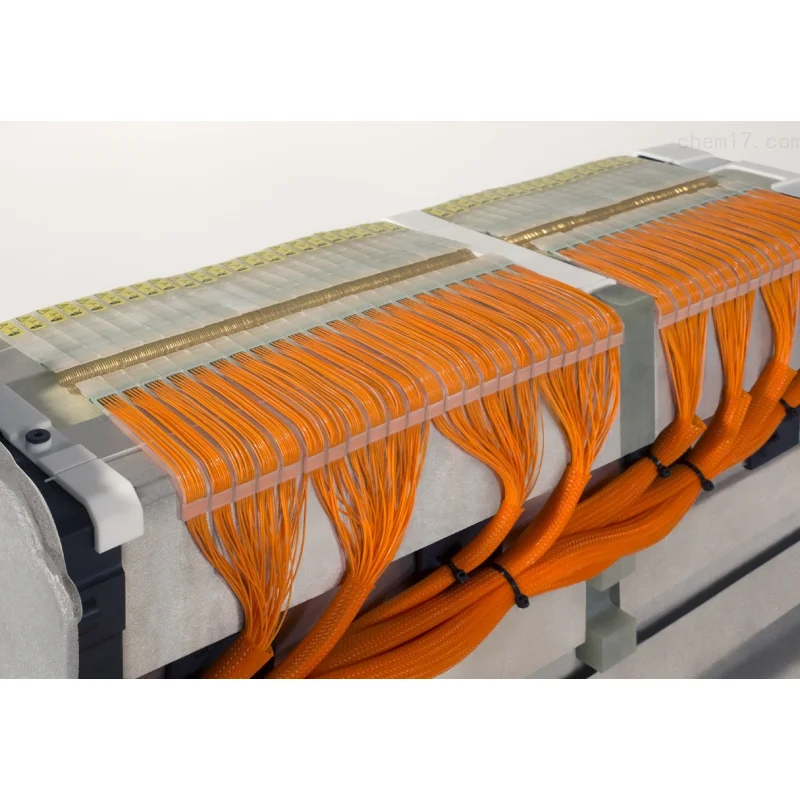
Рентабельный водородный элемент 500 кВт стабильный источник питания Водород для электричества
- Категория: >>>
- Поставщик: WOBO Industrial Group Corp.
Сохранить в закладки 1600844444467:
Описание и отзывы
Характеристики
Hydrogen Fuel Cell Reactor
Cost-Effective Hydrogen Feul Cell 500Kw Stable Power Supply Cell Hydrogen For Electricity
Product Description

Our reactor uses hydrogen as a fuel source to generate electricity in a clean and efficient manner. Unlike traditional power generators, our fuel cell reactor produces no harmful emissions or pollutants, making it an ideal choice for those who prioritize sustainability and environmental responsibility.
At the heart of our product is a cutting-edge proton exchange membrane (PEM) fuel cell, which converts hydrogen into electricity through an electrochemical reaction. Our reactor is designed to be easy to use and operate, requiring only a source of hydrogen and an electrical load to begin generating power. With no moving parts and minimal maintenance requirements, our fuel cell reactor provides a reliable and hassle-free source of electricity for a wide range of applications.
Whether you're looking to power your home, business, or off-grid cabin, our hydrogen fuel cell reactor delivers the performance and reliability you need. With a compact and modular design, our reactor can be easily scaled up or down to meet your specific power requirements. And with a high power density and quick start-up time, our reactor delivers reliable power whenever and wherever you need it.
Invest in the future of energy with the Hydrogen Fuel Cell Reactor - the clean, efficient, and sustainable solution to your power needs.
At the heart of our product is a cutting-edge proton exchange membrane (PEM) fuel cell, which converts hydrogen into electricity through an electrochemical reaction. Our reactor is designed to be easy to use and operate, requiring only a source of hydrogen and an electrical load to begin generating power. With no moving parts and minimal maintenance requirements, our fuel cell reactor provides a reliable and hassle-free source of electricity for a wide range of applications.
Whether you're looking to power your home, business, or off-grid cabin, our hydrogen fuel cell reactor delivers the performance and reliability you need. With a compact and modular design, our reactor can be easily scaled up or down to meet your specific power requirements. And with a high power density and quick start-up time, our reactor delivers reliable power whenever and wherever you need it.
Invest in the future of energy with the Hydrogen Fuel Cell Reactor - the clean, efficient, and sustainable solution to your power needs.
Product Parameter
Product name | Hydrogen fuel cell | |
Number of reactor segments | 420 | |
Rated power | 140kw | |
Peak power | 150kw | |
Operating ambient temperature | -30℃~45℃ | |
Operating ambient temperature | >10000H | |
* Customizing products according to customer requirements. | >10000H | |
Technical Principle

Working principle of Hydrogen fuel cell reactor
Hydrogen fuel cell reactor generates electrical energy by converting the chemical energy stored in hydrogen gas into electrical energy through an electrochemical process. The fuel cell reactor consists of an anode, a cathode, and an electrolyte membrane.
The hydrogen gas is fed into the anode side of the fuel cell reactor, where it reacts with a platinum-based catalyst to produce protons and electrons. The protons pass through the electrolyte membrane to reach the cathode side, while the electrons are forced to flow through an external circuit to reach the cathode. This flow of electrons generates electrical current that can be used to power electrical devices.
At the cathode side, oxygen gas is fed into the fuel cell reactor, and it reacts with the protons and electrons to form water and heat. The water vapor and heat are the only by-products of the reaction, making the fuel cell reactor a clean and efficient energy source.
The key to the fuel cell reactor's operation is the electrolyte membrane, which allows only positively charged protons to pass through while blocking the negatively charged electrons. This membrane separates the anode and cathode, preventing them from coming into contact and shorting out the fuel cell reactor.
Overall, a hydrogen fuel cell reactor is a reliable and efficient energy source that operates through an electrochemical process, with a modular and scalable design that makes it suitable for a wide range of applications.
Product Advantages

Product Characteristics
1.Ultra-thin composite board
2.High power density
3.Low cost, long life
4.High speed voltage inspection
5.Automated mass production
6.Easy to integrate and expand
Product Application Field

Transportation
Fuel cell vehicles (FCVs) use hydrogen fuel cells to power electric motors, offering a zero-emission alternative to conventional internal combustion engines. They can be used in cars, buses, trucks, and even trains and ships.
Portable Electronics
Hydrogen fuel cells can power small electronic devices like smartphones, laptops, and tablets, providing longer battery life and faster charging.
Backup Power
Fuel cells can serve as backup power systems for critical infrastructure, such as data centers, hospitals, and emergency response
centers. They ensure uninterrupted electricity supply during power outages.
centers. They ensure uninterrupted electricity supply during power outages.
Remote Power Generation
In remote or off-grid locations, fuel cells can provide reliable electricity for communities or remote installations, such as weather stations and telecommunication towers.
Stationary Power Generation
Fuel cells can be used as stationary power plants to generate electricity for homes, businesses, and industrial facilities, operating as distributed energy systems.
Material Handling
Hydrogen fuel cells power forklifts and other industrial vehicles, offering longer operating times and faster refueling compared
to traditional battery-powered equipment.
to traditional battery-powered equipment.
Product Show

Related products
Membrane Electrode
Bipolar Plate
Catalyst
Collector Plate
Hydrogen Fuel Cell Titanium Mesh
Nickel Mesh For Hydrogen Fuel Cells
Company Profile

WOBO Group has been engaged in the low-temperature and air separation industries for decades. Operating Low-temperature Application Equipment, Gas Separation Equipment, Green New Energy Equipment and other products. Providing customers with the best products and solutions has always been our goal.
The WOBO Group has engaged in technical cooperation with several large fuel cell stack development enterprises, accumulating rich experience in equipment operation. The company not only possesses a complete system for product research and development and production, but also has honed a highly experienced and capable technical team, dedicated to providing advanced products and quality services to customers. We hope that our meticulous design, excellent quality, and high-quality service can provide a continuous source of power for the development of our customers.
The WOBO Group has engaged in technical cooperation with several large fuel cell stack development enterprises, accumulating rich experience in equipment operation. The company not only possesses a complete system for product research and development and production, but also has honed a highly experienced and capable technical team, dedicated to providing advanced products and quality services to customers. We hope that our meticulous design, excellent quality, and high-quality service can provide a continuous source of power for the development of our customers.


Похожие товары
Сосновый пиломатериал деревянная конструкция обработана Сосновый брус дерева Mgp-10 Сосновый Брус
408,20 $
Высокое качество, соотношение цены и качества, вентилятор радиатора 12 В, пластиковый охлаждающий вентилятор радиатора 600 Вт, Умный вентилятор радиатора OEM A4539064300
27,00 $ - 33,00 $
Высококачественные оконные кондиционеры марки Gree для ВКЛ./ВЫКЛ.
231,00 $ - 255,00 $
49389-05601 SMW251429 Турбокомпрессор для Great Wall Haval H5 H6 4G63S4T 2 0
DRX RPC1919 OEM IP67 жесткий ударопрочный проект переноски рюкзак пластиковый чехол
5,00 $ - 80,00 $
316L хирургическая сталь вогнутая вставка заглушки проушины расширитель инструмент для пирсинга Профессиональные украшения
Emerson/Vertiv Телеком замыкатель постоянного источника питания системы NetSure 531 AC1 серии с модулями R48-2000a3 R48-2000e3 M520S
2 880,00 $ - 2 900,00 $
Новые поступления
Новинки товаров от производителей по оптовым ценам
OrionStars онлайн-машина для рыбалки и свинга высокоприбыльная монетная игра США оптовая продажа агент с высокой прибылью Разыскивается
Рекламный надувной самолет украшение для костюма самолета ходячие костюмы продажи
C100 % восковая ткань с принтом китайские поставщики поставляют настраиваемые печатные африканские восковые ткани
0,93-0,97 $
Прямые продажи с фабрики могут быть оптовые складные тележки для покупок Удобные и уличные продуктовые
8,39-9,89 $
Hantek щупы осциллографа автоматический Тестовый Кабель Ht30a Bnc к банану двойная Банановая головка многоцелевое автомобильное
3,10-3,44 $
Горячая распродажа ручная работа традиционная деревянная урна для домашних животных квадратная коробка золы собак и кошек персонализированная Лучшая цена
Промышленная серия CL Бесшумная цепь стальная зубчатая Фланцевая контактная звездочка соединяющая бесшумные цепи
1-1,50 $