Alpha 1 microglobulin реагент для диагностики IN Vitro от производителя
- Категория: >>>
- Поставщик: Shijiazhuang Hipro Biotechnology Co. Ltd.
Сохранить в закладки 60767619737:
Описание и отзывы
Характеристики
Package Insert
Hipro™ α1-MG Test
(Rate Scattering Turbidimetric Method)
[Product Name]
General name:alpha-1-microglobulin Test Kit
(Rate Scattering Turbidimetric Method)
Trade Name:α1-MG test
[Intended Use]
This product is used to determine the content of alpha-1-microglobulin(α1-MG) in human serum.
[Principle]
The antibody of alpha-1-microglobulin is coated on the latex surface. The α1-MG in the sample and the antibody become to immune complexes by Latex agglutination reaction. The immune complexes will produce the phenomenon of light scattering which is proportional to the intensity of scattered light and samples of α1-MG levels. Using specific protein analyzer to measure the intensity of scattered light, the concentration of α1-MG is determined by comparing the turbidity of samples to the standard concentration.
[Packing of the Kits]
Name | Quantity and specification | |||
1 test | 25 tests | 50 tests | 100 tests | |
R1 | 300μl×1 | 300μl×25 | 300μl×50 | 300μl×100 |
R2 | 50μl×1 | 50μl×25 | 50μl×50 | 50μl×100 |
[Main Components]
| Component | content |
Reagent 1 | Phosphate buffer (pH 7.5) | 50mmol/L |
Polyethylene glycol 6000 | 5.8% | |
Reagent 2 | Anti-human α1-microglobulin antibody latex particles | 5g/L |
[Storage and Validity Period]
Store at: 2~8℃
Validity Period: 1 year
[Suitable Machine]
This product is suitable for HP-083/4-I, HP-083/4-II, HP-AFS nephelometry immunoassay analyzers.
[Sample Requirements]
Fasting blood collection and separation of serum as soon as possible; avoid hemolysis; store at 2~4℃ for 3 days.
[Testing Procedure]
Patient sample | 5μl |
Reagent (R1) | 300μl |
Mixing | |
Reagent (R2) | 50μl |
Mixing, and then put the reaction cup into the test channel. The analyzer will test and display the results automatically. | |
[Reference Range]
<12mg/L
Recommended that each laboratory establish its own reference range
[Limitations of the Method]
1. This product is based on latex agglutination system, only suitable for the specific analyzer.
2. This result is for clinical reference only; comprehensive consideration should be combined with the clinical management of patients with symptoms / signs, medical history, other laboratory tests and treatment response.
3. When hemoglobin≤10g/L, triglyceride≤6.5mmol/L, or bilirubin≤310μmol/L, it has no interference to this determination
[Technical Parameter]
Linearity range: 5mg/l~90mg/l,r≥0.990
Accuracy: recovery rate±10%
Limit of detection: 3mg/l
Within-batch Precision: ≤10%
Batch-batch Difference: ≤15%
[Precautions]
1,This kit is only for IVD.
2,If the regent is splashed on the skin or eyes, wash with water.
3,Do not use the kits beyond shelf life. Do not mix different batches of reagents.
[References]
Shang Hong, Wang Liu three, Shen Ziyu and so on. National Clinical Laboratory Practice (Fourth Edition) [M] Beijing: People's Medical Publishing House,2015:224-225.
Manufacturer: Shijiazhuang Hipro Biotechnology Co.,ltd
Address :No.3 Building,Block C,Fangyi Tech. Park, No. 313Zhujiangdadao Street,Shijiazhuang,050035 China
Tel: 86-0311-83855889
Fax: 86-0311-83859087
EU Representative:
Name: Lotus Global Co., Ltd
Address: 15 Alexandra Road, London, NW8 0DP, UK
[SYMBOLS USED]
Description | |
| Use By |
| Batch Code |
| Manufacturer |
| Keep Away from Sunlight |
| Temperature Limitation |
| In Vitro Diagnostic Medical Device |
| Authorized Representative in the European Community |
| CE Mark |
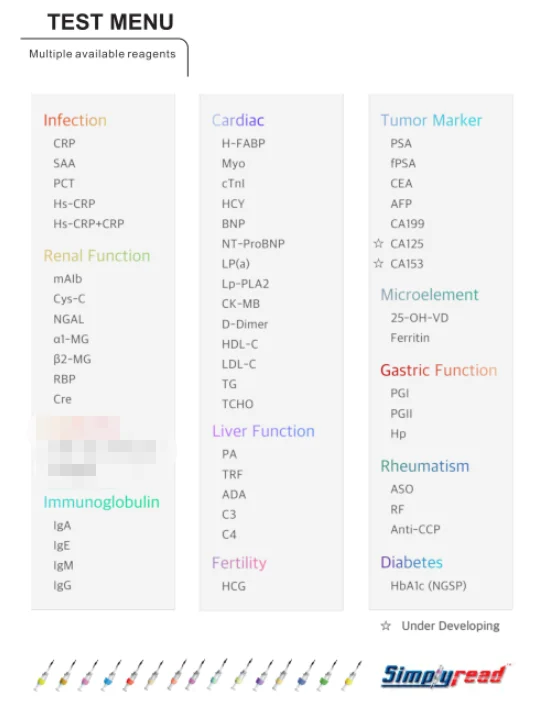
About Hipro
Hipro biotechnology Corp, founded on September 29th, 2006, is a high-tech enterprise based on international advanced medical technologies and brilliant self-innovations to provide first class products all over the world. Hipro focuses on R&D, manufacturing, marketing, and relevant services of Point-of Care products. Hipro, with her R&D center in Silicon Valley US, and production base in Hebei Province, has established branches in San Francisco, Beijing, Suzhou, Chengdu, Guangzhou,Mumbai,etc., and launched 3.69 acres industrial park project in China Medical City.








Packing: We use high-strength and environmental protection material to pack our product. The packaging can protect the goods from dropping from 20 meters high place.

Shipping:



or other air transportation.
Похожие товары
Сладкая жизнь, обычный тип, 24 месяца, упаковка манго, оптовая продажа, замороженное полуобрезанное манго Lifefoods из Вьетнама
Хорошая цена, мощность аккумулятора 3 тонны, плоская электрическая тележка с дистанционным управлением
Набор измерительных приборов из нержавеющей стали, набор ложек для выпечки, для сухого и жидкого ингредиента, цвет под заказ
Акция King Long Used Ev Bus роскошные новые энергосберегающие городские автобусы и трехфазные Bev-автобусы 268 Hp моторные автобусы на продажу
Heavybao коммерческий склад большегрузных транспортных средств 4 колеса ручная тележка для пуш-ап платформенная ручной тележки
Клапан EGR подходит для Land Rover Discovery 4 2010-2016 3.0L TDV6 LR018753 LR063122, правосторонний аккумулятор GL2229, автомобильные запасные аксессуары
LF 125 кГц проксимити RFID смарт-кард-ридер для ID-карты клавиатуры ID card reader plug and play драйвер уменьшенного использования пластин кард-ридер
Новые поступления
Новинки товаров от производителей по оптовым ценам