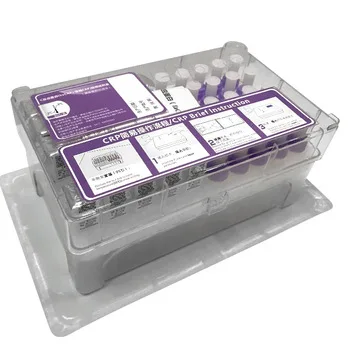
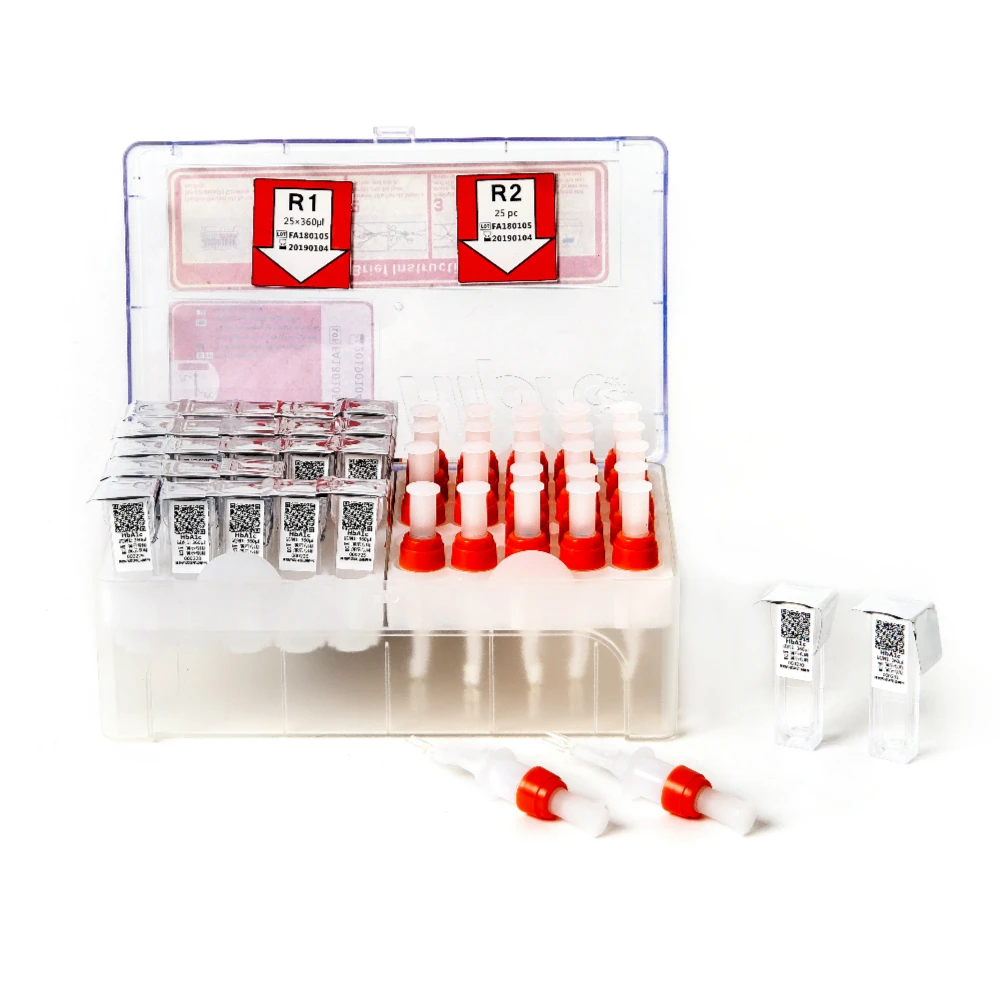
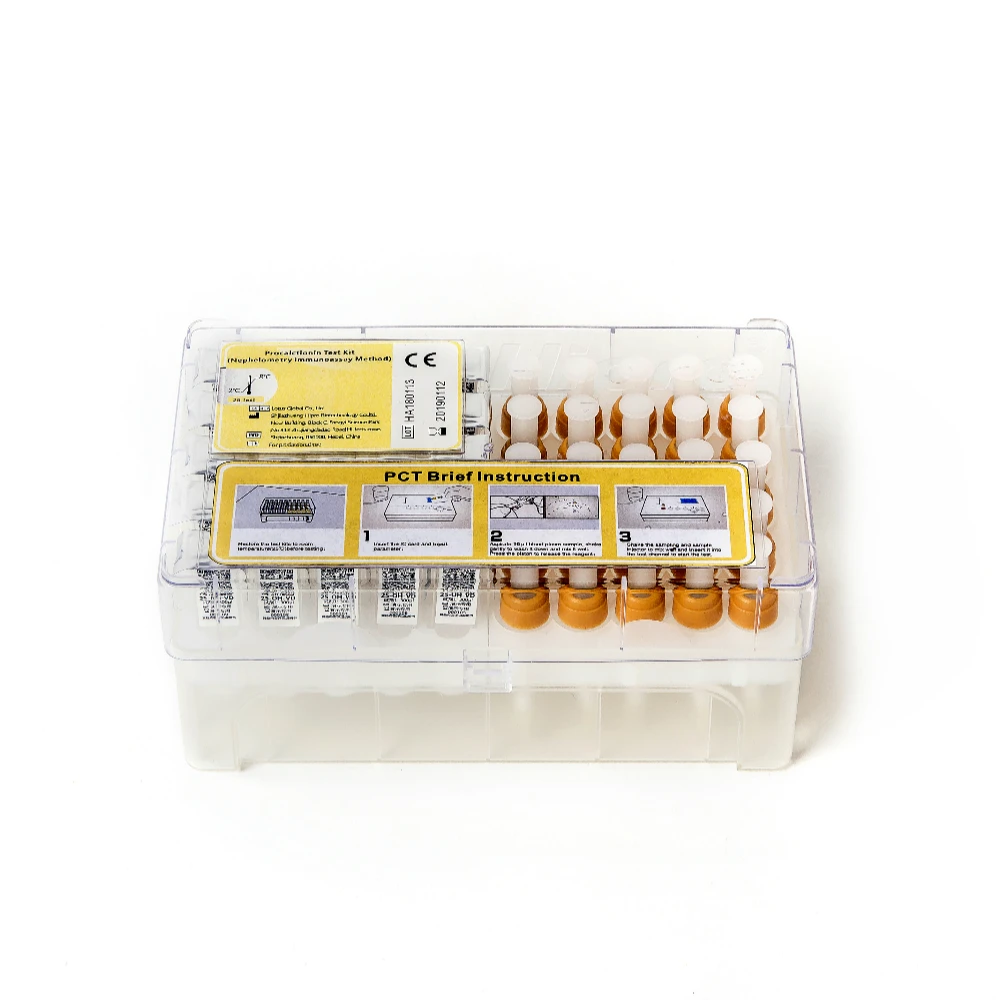
Набор для тестирования витамина D
- Категория: >>>
- Поставщик: Shijiazhuang Hipro Biotechnology Co. Ltd.
Сохранить в закладки 62385149357:
Описание и отзывы
Характеристики

Product Name
General Name : 25-hydroxy-Vitamin D Test Kit (Immune Fluorescence Detection Method)
Trade Name:Hipro™ 25-OH-VD Test
Intended Use
This product is used to determine the content of 25-hydroxy-Vitamin D (25-OH-VD) in human serum, it is mainly used for the auxiliary diagnosis of vitamin D deficiency related diseases.
Principle
The 25-hydroxy-Vitamin D in the sample and the monoclonal antibody in reagent 1 become to immune complexes. The antigenic determinant-DNA coupling template in Reagent 2 binds to the remaining monoclonal antibodies, and the unbound antigenic determinant-DNA coupling template and dNTPs are used to synthesize double-stranded DNA products under the action of polymerase. This product binds to fluorescent dyes and will produce fluorescence which is proportional to the intensity of fluorescence and samples of 25-OH-VD levels. Using specific protein analyzer to measure the intensity of fluorescence, the concentration of 25-OH-VD is determined by comparing the fluorescence intensity of samples to the standard concentration. The kits contains all the reactive reagents.
Quantity and Specification
25 Tests/kit , then put into the foam box , with ice bag inside to keep the suitable temperature to protect the antibody 's activity.
Storage and Validity Period
Store at: 2~8℃
Validity Period: 1 year
Suitable Machine
This product is suitable for HP-AFS / 1 , HP-AFS / 3 and HP-083/4-I, HP-083/4-II nephelometry immunoassay analyzers.
Sample Requirements
Fasting blood collection and separation of serum as soon as possible; avoid hemolysis.
Collect sample
Insert into cuvette
Insert into instrument

Reagents matched with Instrument uses
* 38 existing reagents for testing and more to be amplification.
* Different from test strip , direct liquid phase , analysis more accuracy.
* Simply Read Patented Integrated Sample Collector, automatic sample adsorption and preloaded reagents, operation more simplicity.
* System calibration by custom quality control supplied by Bio-Rad / Randox.

*Total Laboratory Automation Immunoassay Analyzer HP-AFS / 3

About Hipro
Hipro biotechnology Corp, founded on September 29th, 2006, is a high-tech enterprise based on international advanced medical technologies and brilliant self-innovations to provide first class products all over the world. Hipro focuses on R&D, manufacturing, marketing, and relevant services of Point-of Care products. Hipro, with her R&D center in Silicon Valley US, and production base in Hebei Province, has established branches in San Francisco, Beijing, Suzhou, Chengdu, Guangzhou,Mumbai,etc., and launched 3.69 acres industrial park project in China Medical City.
Hipro has a world-class high capacity manufacturing facility (10000 sq.m.) with 1500 sq.m. current GMP plants and strict quality control system. The quality system is ISO 13485 and CE certified by TUV Rheinland.
The sales network covers the whole Chinese mainland, and throughout the world: US, Canada,Korea,India,Turkey,Epypt and more than 30 countries.
Jake Jiang
Sales Manager
Mobile / Whatsapp: +86 186 3305 3287
Wechat / Skype: jakeagn
Похожие товары
Медицинский диабетический тест на ногу, нейлоновая монофиламентная линия
Медицинский класс, бестселлер, брелок, набор для быстрого тестирования Кала на скрытую кровь для пациента с CE
Lh Тест Овуляции кассетные овуляционные тесты для дома и больницы
IClean оптовая продажа, один шаг, медицинский быстрый диагностический тест на малярию, набор для тестирования на малярию
Распродажа, набор для быстрого тестирования ДНК для собак и домашних животных для одноразового теста
Контекс ECG1212G ekg машина 12 свинцовый ЭКГ тележка машина
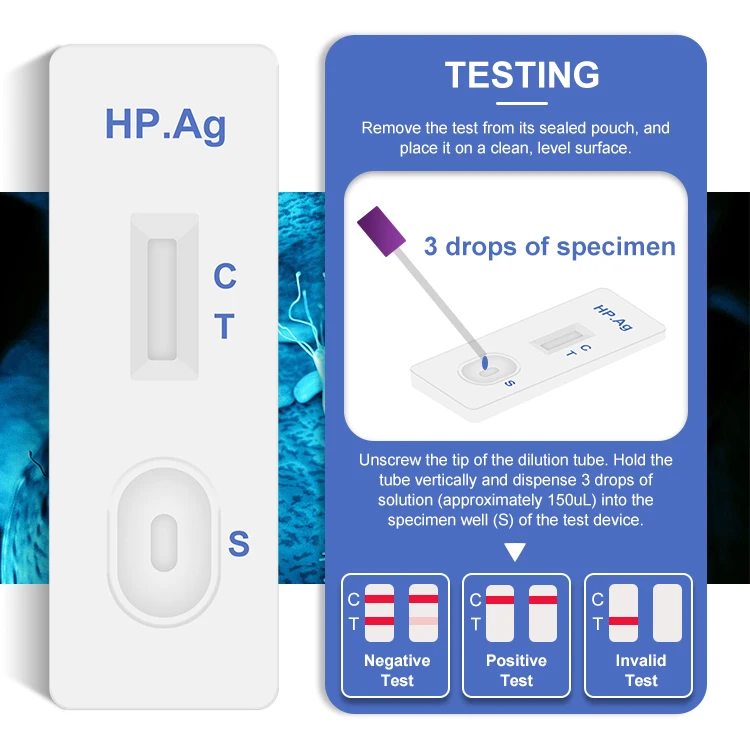
Safecare Hp Ag быстрый тестовый комплект антиген H.pylori диагностический Быстрый самотестирующийся комплект
Новые поступления
Новинки товаров от производителей по оптовым ценам