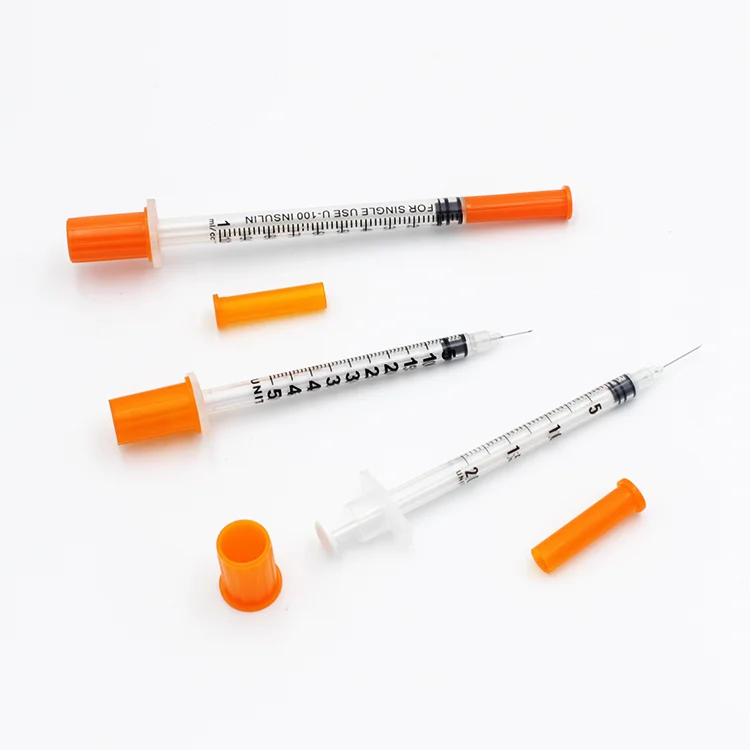
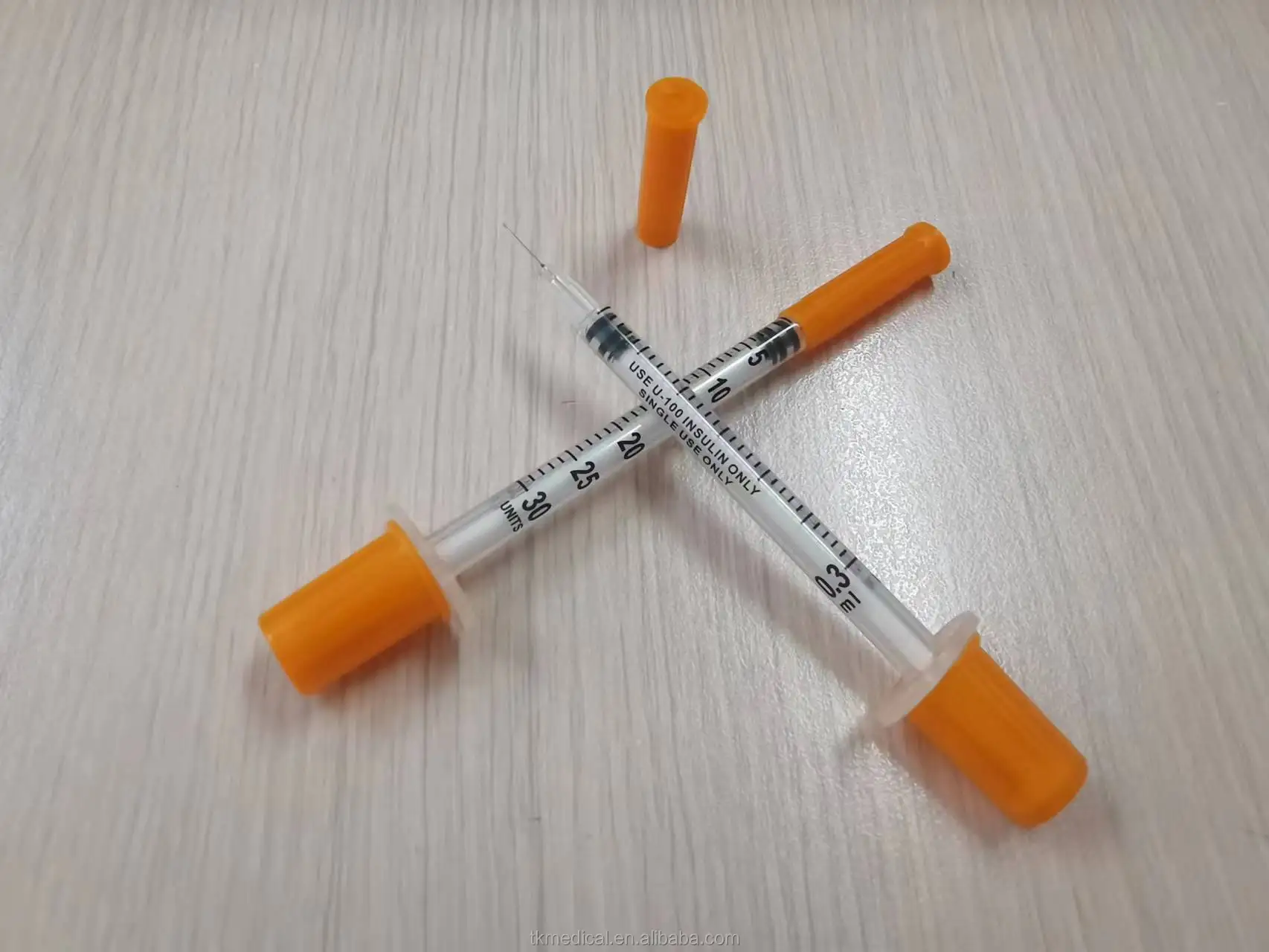
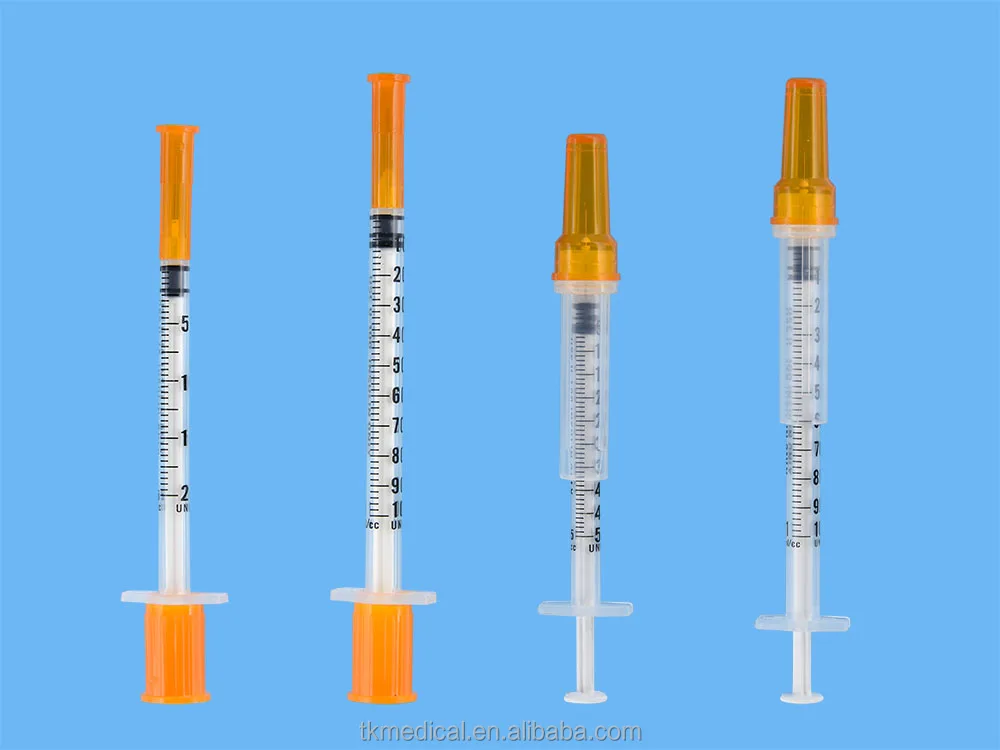
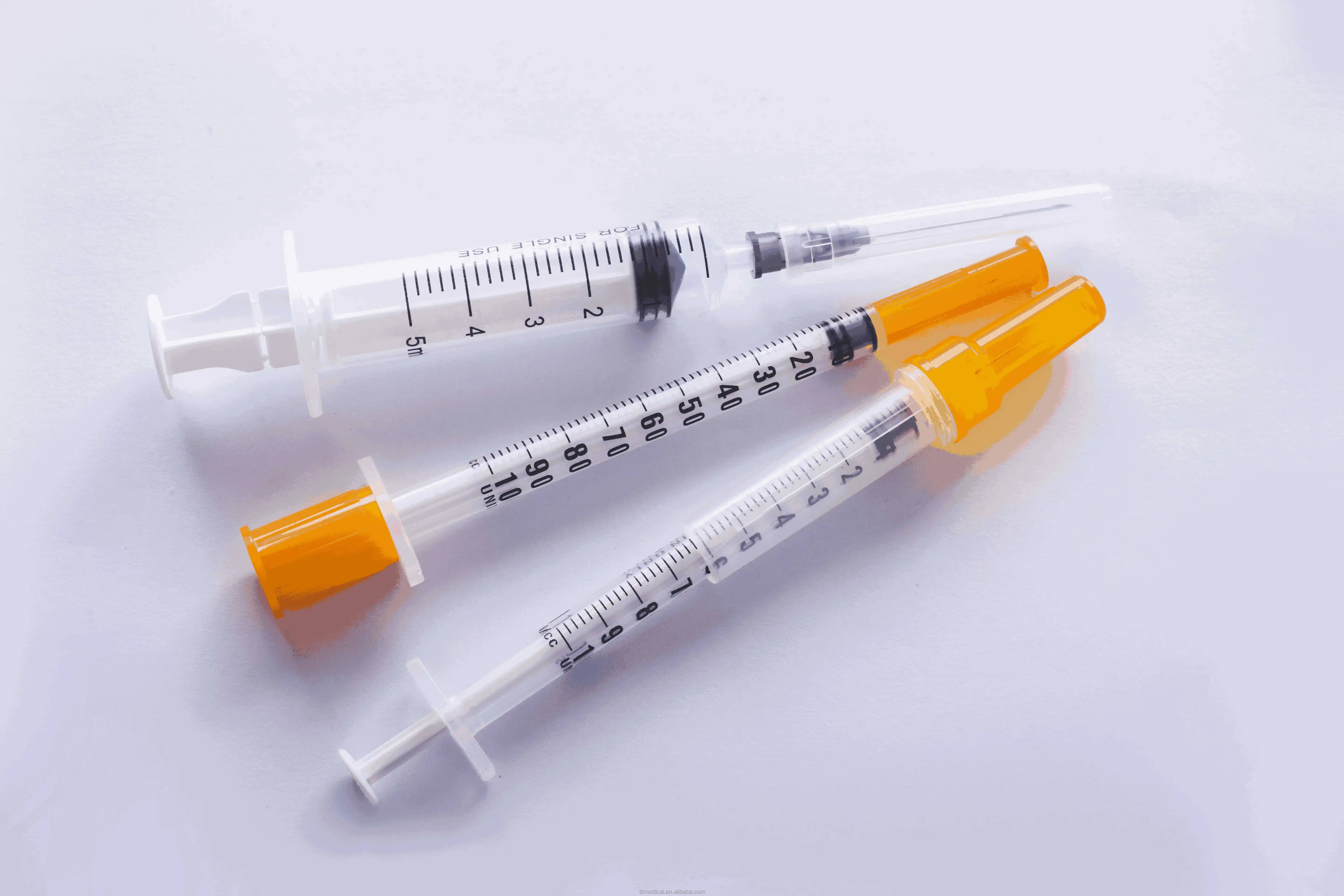
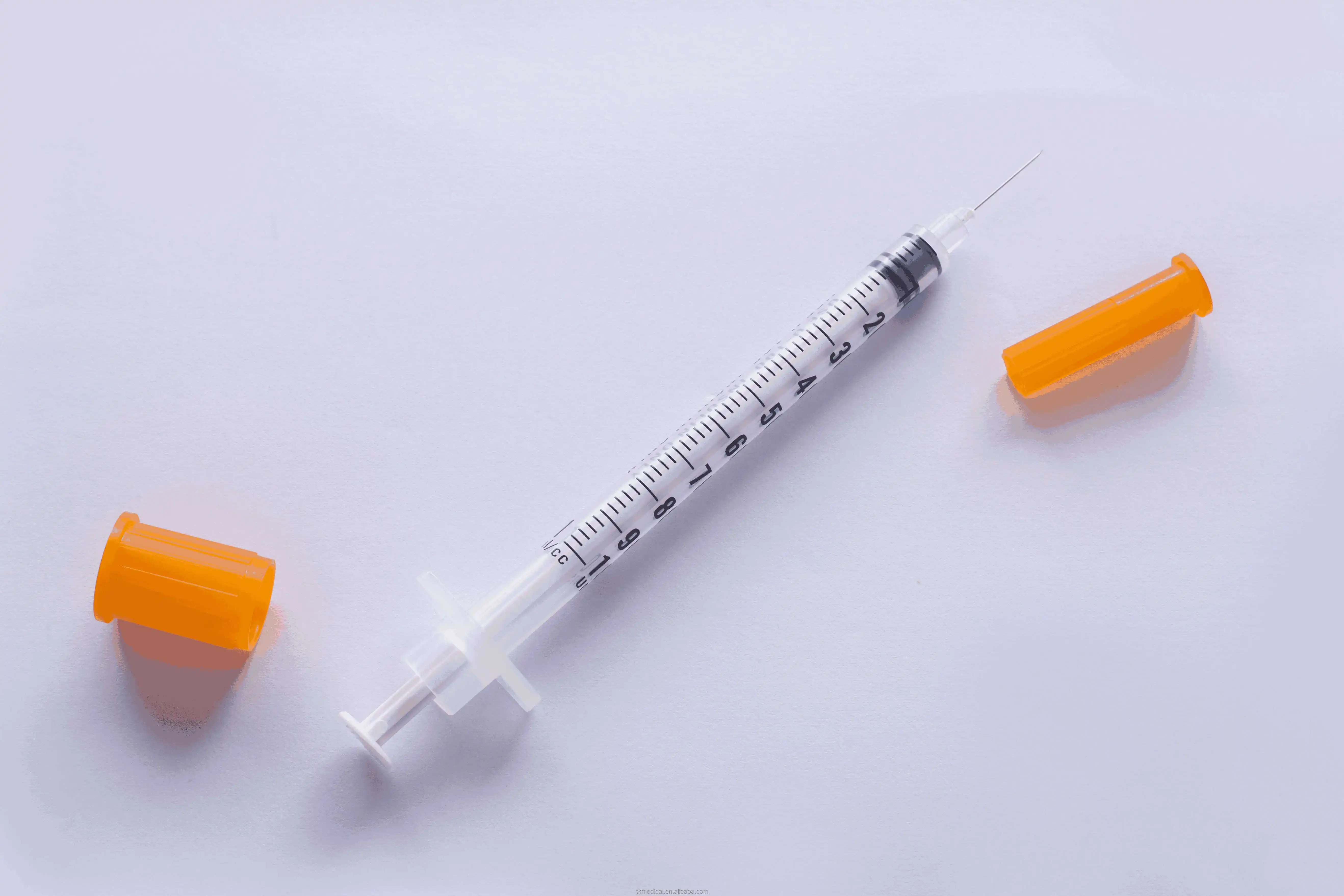
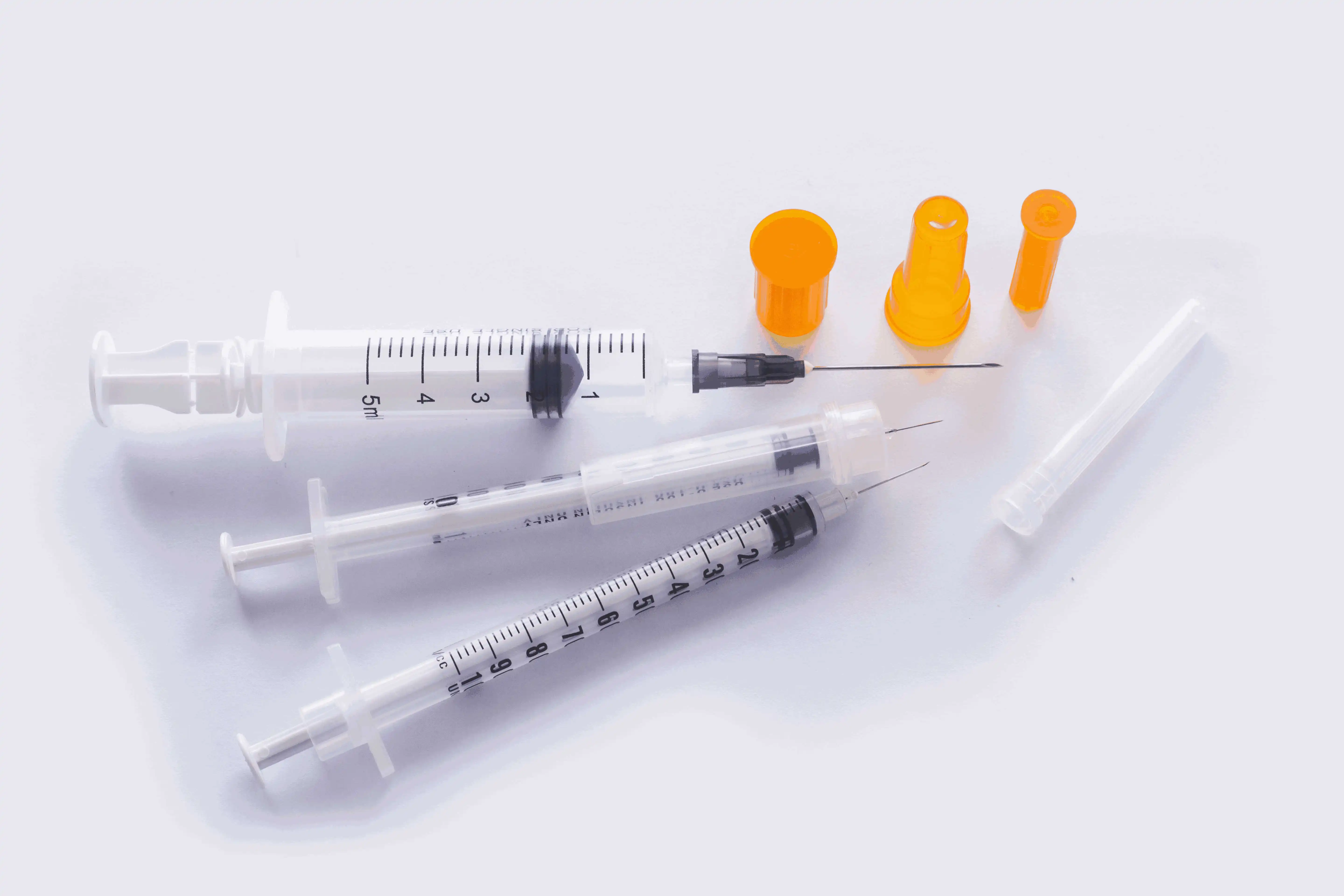
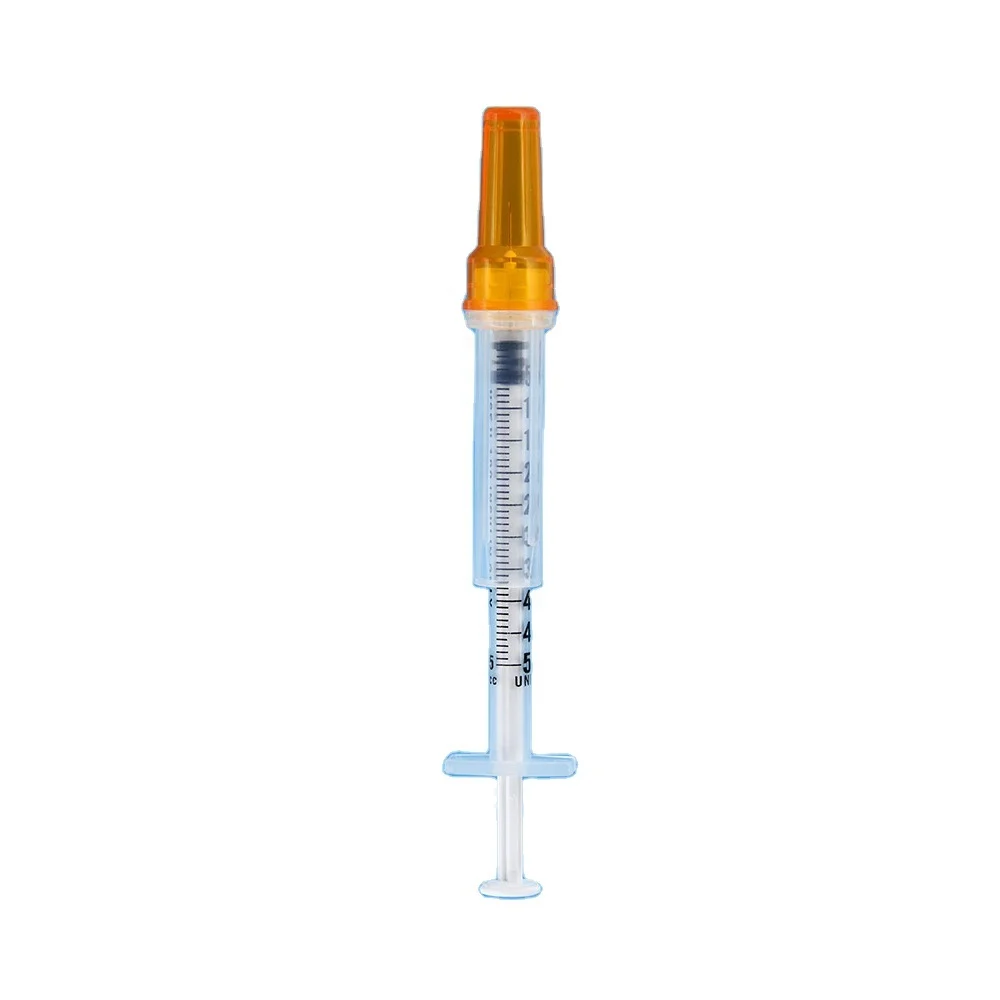
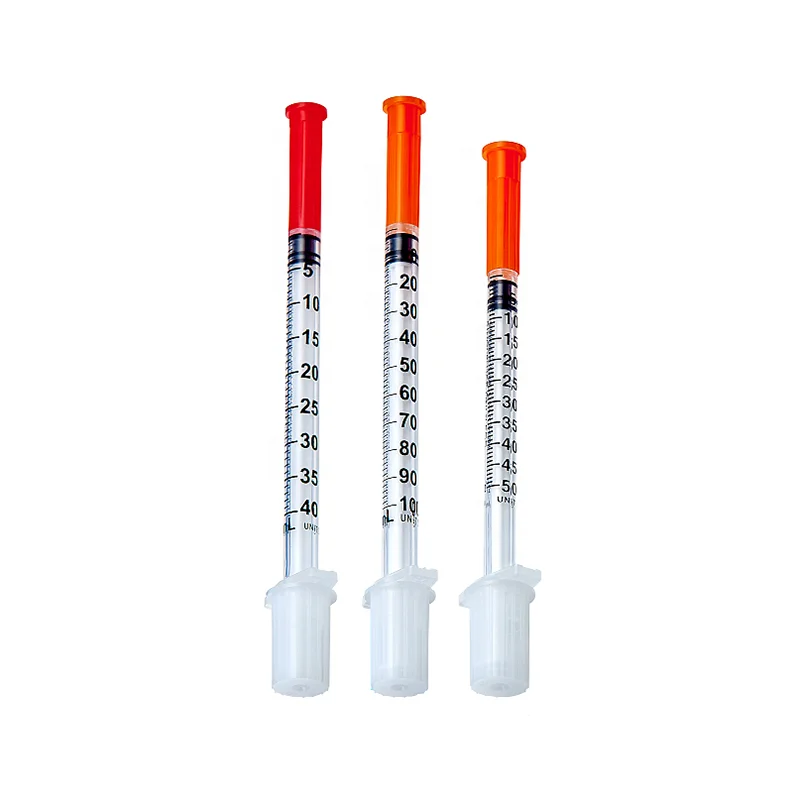
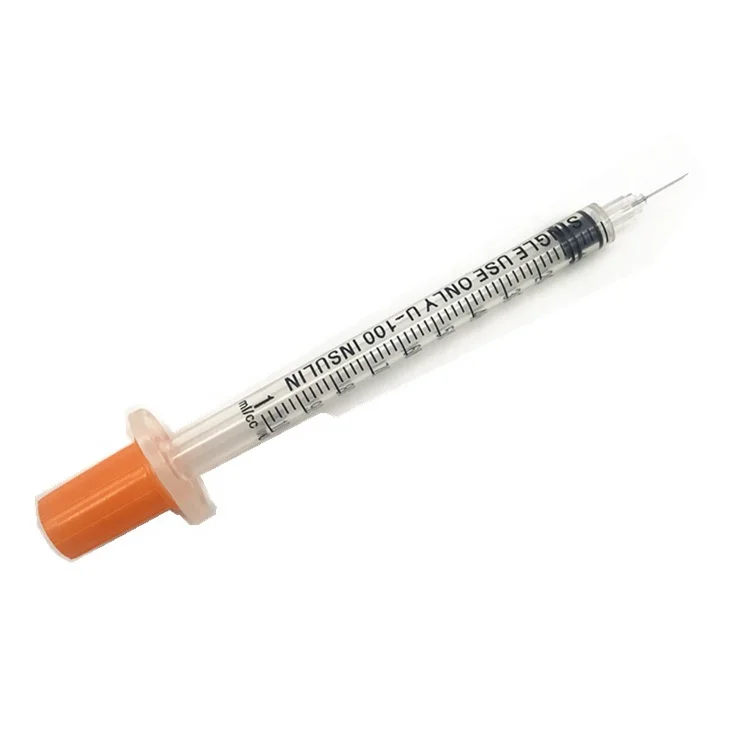
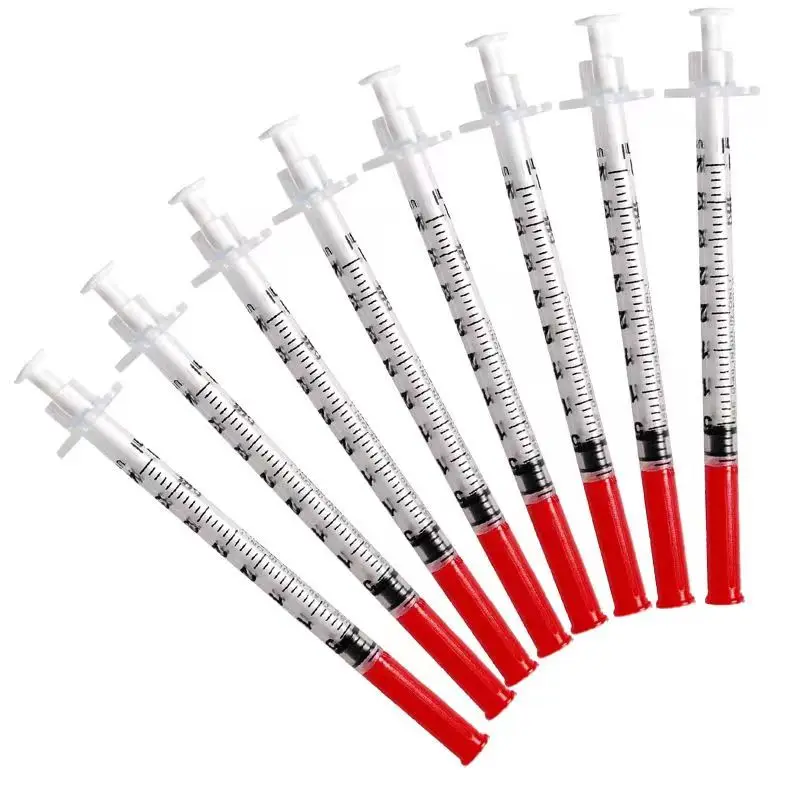
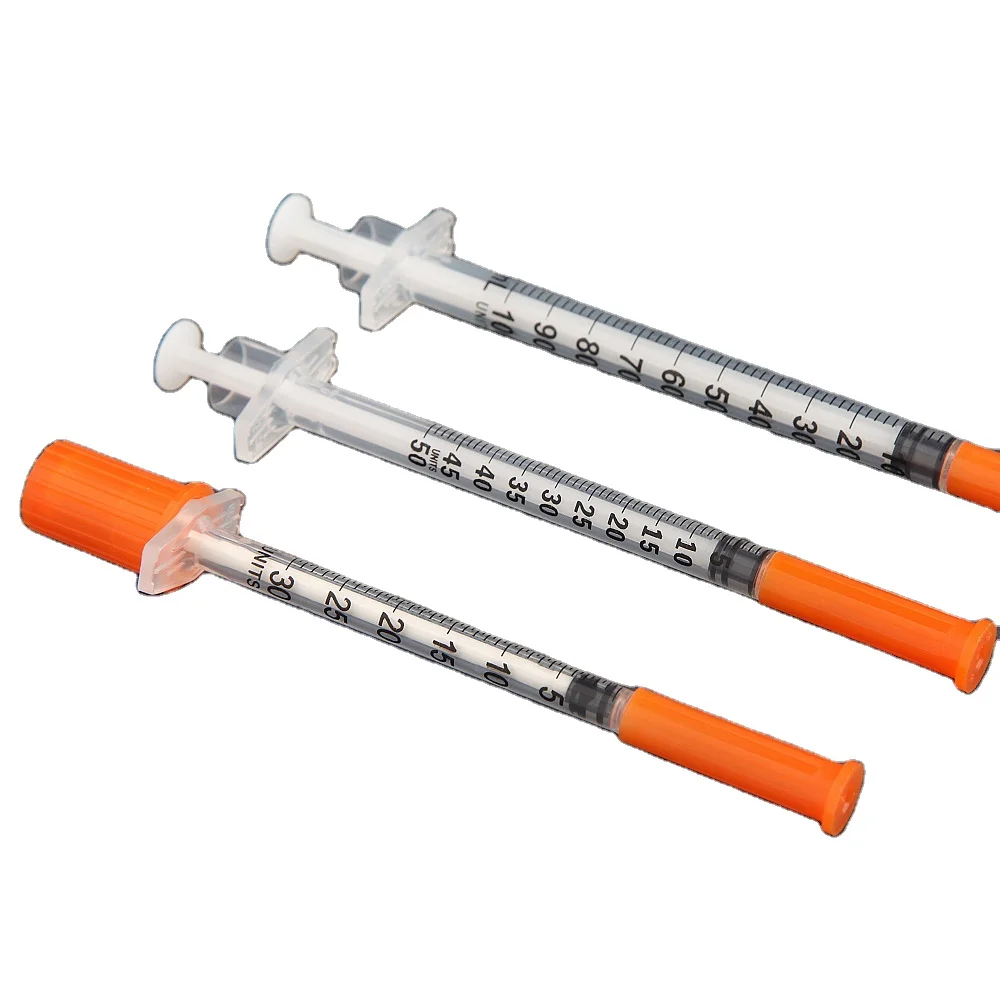
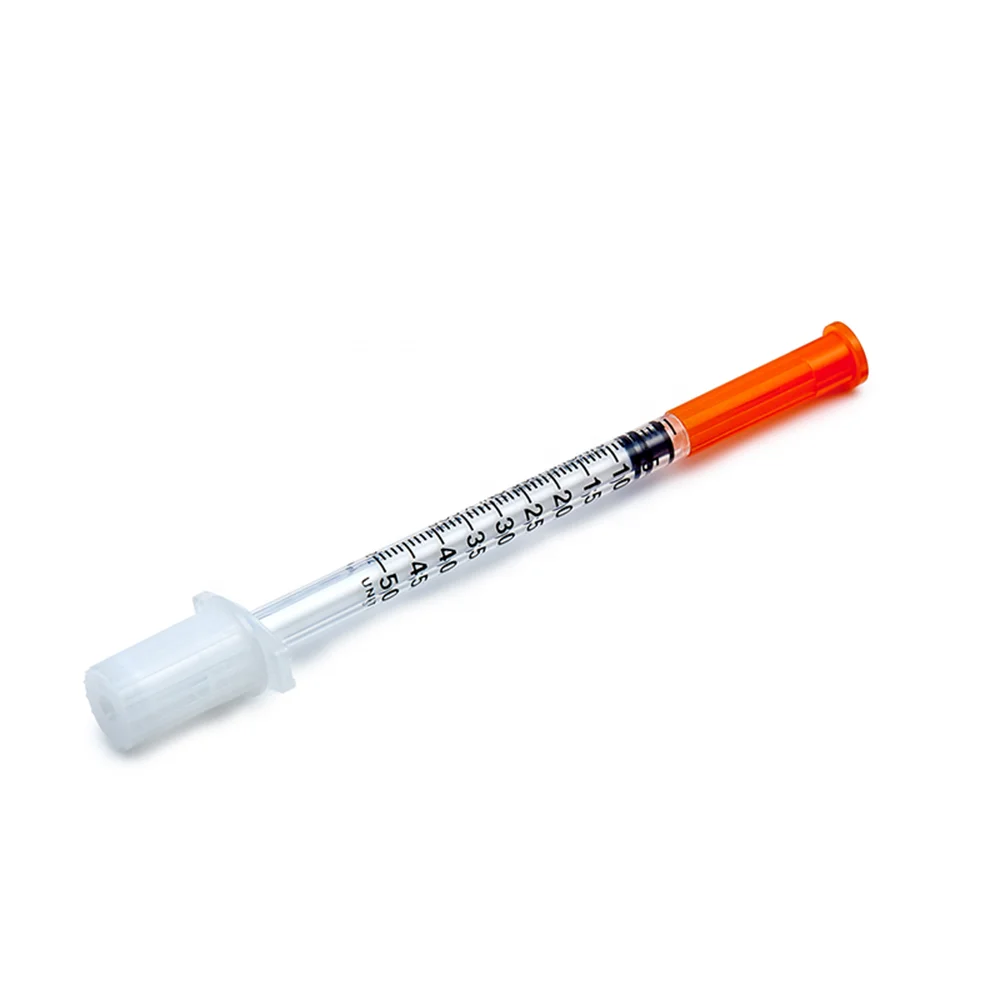
0.3ml Insulin Syringes with needle 29G/30G/31G/32G/33G/34G
- Category: >>>
- Supplier: Anhui Tiankang Medical Technology Co. Ltd.Anhui Ltd.
Share on (1600599548887):
Product Overview
Description
Product Description
Insulin Syringes, specially take insulin for diabetics.
Product packaging
Small Package:PE/ Blister
Medium Package:Medium box/Medium PE bag
Outer Package:Carton


1ml
PE packing:
190x40x0.1mm
18x17x10.7cm / 100 pcs(medium box)
55.5x37.5x35.5 cm / 2000 pcs(carton)
Blister packing:
173x33mm
17.3x15x12.3cm / 100pcs(medium packing)
63.5x365x31.5cm / 2000pcs(carton)
Certifications

Company Profile

Anhui Tiankang Medical Technology Co.,Ltd (Stock Abbreviation: Tiankang Medical, Stock Code: 835942)located in Tianchang City which is rated as the pearl of Anhui.Our company have got official new three board on March 3, 2016 and now we have a registered capital of 57.78 million RMB yuan. We specialize in developing and producing products like safety syringes with retractable needles, auto disable syringes, I.V sets ,T.V sets ,extracorporeal blood circuits for haemodialysers,oral syringes, I.V catheter and intravenous infusion sets with burette etc.
Our company has a factory of more than 600 acres holding a large scaled 100,000 class clean workshop 30,000 square meters.And we now have a staff of one thousand one hundred in including 430 technical engineers of middle and high ranges(about 39% of all the staff). Besides, we now have more than 100 first-class injecting machines and affiliated equipments of assembling and packing. What deserves to be mentioned the most is that we are authorized European CE certificate (in 2002),ISO13485 certificate (in 2002) and WHO—PQS certificate (in 2010) which we hold as passports to enter international market. Our company's products began to be registered with the U.S. Food and Drug Administration in 2012 and currently rank among the best in the country for U.S. Food and Drug Administration-listed products. Autonomic products: safety syringes with retractable needles which registered by U.S. Food and Drug Administration 510 (k), disposable syringes and disposable hypodermic needles also passed U.S. Food and Drug Administration registered 510 (k). From June 6 ,2016 to June 9, with zero defect through the U.S. Food and Drug Administration field QSR 820 audit at the U.S. Food and Drug Administration site.
Factory view




National government leaders visiting




Exhibition

Our company’s products have won orders of domestic large and medium-sized medical institutions and hospitals and they are also sold well aboard;for example,in countries and regions of Europe,Africa and Latin America etc.
The company's syringes of "SAN ZHONG" brand is a famous-brand in Anhui Province, "SAN ZHONG"brand at the same time as Anhui famous trademark.
Since the establishment of the company, relying on the excellent product quality, professional marketing services, Tiankang brand value continuously improved, the market continues to expand. At present, we have developed a series of new products that are easy to use, safe and practical, such as disposable sterilized self-destructing fixed-dose vaccine syringes, disposable sterile safety syringes with retractable needles, disposable intravenous needles etc. Once on the market, products have won wide acclaim from healthcare professionals and patients. Disposable auto disable syringes exports ranked first in the United Nations agencies is the only Chinese supplier shortlisted products.
The company's syringes of "SAN ZHONG" brand is a famous-brand in Anhui Province, "SAN ZHONG"brand at the same time as Anhui famous trademark.
Since the establishment of the company, relying on the excellent product quality, professional marketing services, Tiankang brand value continuously improved, the market continues to expand. At present, we have developed a series of new products that are easy to use, safe and practical, such as disposable sterilized self-destructing fixed-dose vaccine syringes, disposable sterile safety syringes with retractable needles, disposable intravenous needles etc. Once on the market, products have won wide acclaim from healthcare professionals and patients. Disposable auto disable syringes exports ranked first in the United Nations agencies is the only Chinese supplier shortlisted products.
Shippment



We Recommend
New Arrivals
New products from manufacturers at wholesale prices